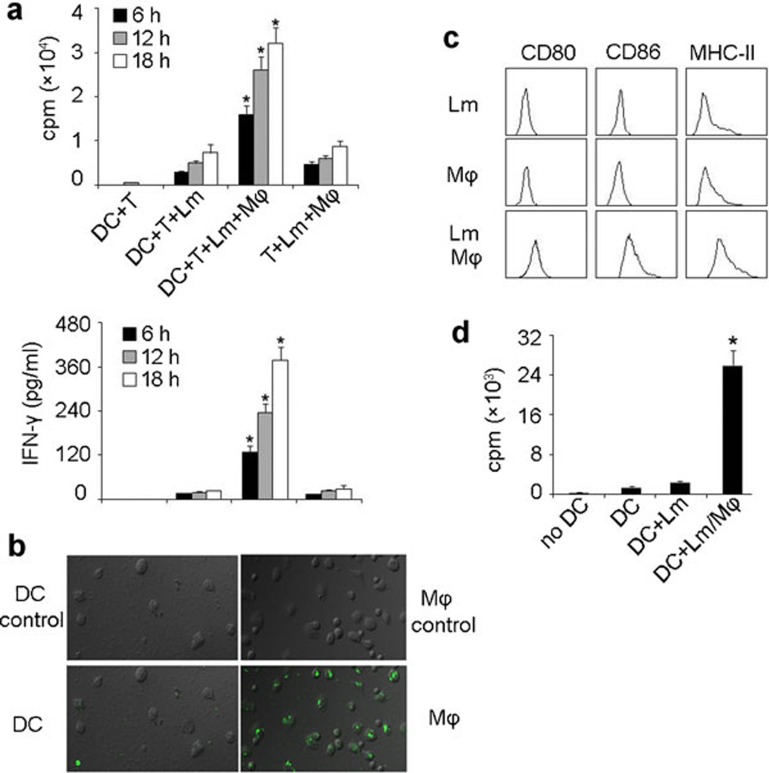
Figure 2.
Macrophages are involved in DCs presenting Lm antigen in vitro. (a) Macrophages were required for DC-elicited Lm-specific T-cell proliferation. Bone marrow-derived DCs (BALB/c background) were incubated with viable Lm (100 µg/ml gentamycin added 30 min later) in the presence or absence of peritoneal macrophages for various time intervals (6, 12 and 18 h). 1×105 splenic T cells, isolated from the spleens of Lm-infected BALB/c mice, were cocultured with those DCs (1×104) or Lm-infected macrophages. T-cell proliferation was measured 72 h later by incorporation of [3H]-thymidine (top) and the supernatants were used for IFN-γ detection by ELISA kit (bottom). *P<0.05, compared with the other corresponding groups. (b) Lm was poorly taken up by DCs. DCs (left) or peritoneal macrophages (right) were incubated with CFSE-labeled Lm for 1 h and the uptake of bacteria was detected by fluorescent microscopy. The top was shown as the background fluorescence. (c, d) DC maturation and presenting of Lm antigens were conferred by the supernatants from Lm-infected macrophage cell cultures. DCs were incubated with the supernatants of Lm-infected macrophages, uninfected macrophages or Lm alone for 24 h. After incubation with Fc receptor-blocking Ab, cells were stained with CD80, CD86 and MHC class II mAbs, respectively, and analyzed by flow cytometry (c). Meanwhile, T cells, isolated from the spleens of Lm-infected BALB/c mice, were incubated with DCs that had been treated with the supernatants of Lm-infected macrophages, Lm-infected DCs or untreated DCs. T-cell proliferation was measured 72 h later by incorporation of [3H]-thymidine (d). Experiments were repeated at least three times. *P<0.05, compared with macrophage-absent groups. DC, dendritic cell; IFN, interferon; Lm, Listeria monocytogenes; Ab, antibody.