Abstract
Brain and eye tissues are subject to a reduced version of immune surveillance, which has evolved to protect the particularly sensitive tissues from accidental bystander damage created by regular inflammatory responses. Yet, there are autoimmune diseases in both organs. This review discusses the nature of immune reactivity in the healthy eye and brain tissues, and mechanisms that can overcome the protective barriers to create tissue specific disease.
Keywords: autoimmunity, autoantigenic strength, immune privilege, blood-brain barrier (BBB), blood-retina barrier (BRB), anterior chamber, T cells, experimental autoimmune encephalomyelitis (EAE), experimental autoimmune uveitis (EAU), central nervous system (CNS), eye
Introduction
The immune system is programmed to continuously scan the body for potentially perilous microbial organisms. In doing so, elements of the immune system identify suspicious structures, and report the information to organized immune organs, where responses are mounted in order to neutralize the potential aggressors. However, immune surveillance is not absolute, and it does not function equally well in all organs. In particular, and on first sight against intuition, some of the most complex and vulnerable organs, including the central nervous system (CNS), the eye and the testis, seemed to be ignored by the immune system, a neglect euphemistically termed “immune privilege”1.
Unfortunately, the immune privileged status of the CNS and eye does not provide absolute protection against immune-mediated diseases. Multiple sclerosis (MS), the most important inflammatory brain disease among younger people in moderate climate zones, seems to be an autoimmune disease, caused by brain-autoreactive T lymphocytes. After being activated in the peripheral immune system, these T cells intrude into the brain, and there attack local tissue. This concept is not formally proven, but it rests on several lines of evidence. Indeed, besides signs of tissue degeneration, freshly active MS lesions show immune cell infiltrates, which suggest on-going active autoimmune processes. In addition, the risk of disease is linked to a growing number of genes, so far all related to immune response and inflammation. Furthermore, the most efficient current therapies for MS are immunomodulatory, ie, they alter ongoing acute immune responses. Finally, in recent animal models, spontaneously developing autoimmune demyelinating CNS disease is clearly immune-mediated and resembles early phases of human MS.
Autoimmunity also occurs in the eye. Autoimmune inflammation of the middle layer of the eye, the uvea, is not uncommon in human populations. Uveitis is a sight-threatening, intraocular inflammatory disease in humans and is associated with a high visual morbidity. Like MS, there are diverse lines of evidence indicating an autoimmune pathogenesis in uveitis. Animal models of uveitis, collectively termed experimental autoimmune uveitis (EAU), have been developed in susceptible rodents, mostly by immunization with ocular self-antigens or by adoptive transfer with uveitogenic T cells2. A number of uveitogenic molecules have been characterized, of which the interphotoreceptor retinal binding protein (IRBP) is uveitogenic for both mice and rats. Established animal models of acute, recurrent and chronic EAU provided us with the opportunity to examine differences in the mechanisms of disease, and determine the molecular and cellular properties that lead to disease progression.
In this brief review we shall discuss some structural and functional features that contribute to the immune privileged status of the CNS and eye, and how the privileged status can be lost, giving rise to local autoimmune disease.
Immune privileged tissues
The CNS and eye differ from other organs due to several structural peculiarities that profoundly affect local immune surveillance. In particular, the nervous tissues are secluded from the blood flow by a specialized, tight barrier comprising specialized endothelial cells that admit only a selected and tightly controlled set of circulating blood components into the CNS. Secondly, the CNS is less efficiently drained by lymphatic vasculature than other tissues. Hence the transport of antigenic material, either soluble, or transported by dendrite cells to peripheral immune organs is markedly reduced. Finally, CNS tissue lacks many of the functional cues, such as MHC molecules, cell adhesion molecules, chemokines and cytokines, which together create a hospitable milieu for T lymphocytes, allowing proper immune function and long-term survival.
However, it should be noted that the immune privileged status of the CNS refers to a deficiency of the adaptive immune system. In marked contrast, the innate immune reactivity of CNS tissues is ensured by a tight network of microglia cells, bone marrow-derived phagocytes, which essentially cover the entire CNS tissue with fine processes that permanently seem to scan the surrounding microenvironment for alarming changes. Indeed, any unusual event, whether intrusion of a microbial agent, traumatic or spontaneous degeneration of parenchymal cells, or neoplastic transformation is sensed by the microglia cells and results in activation.
The blood brain barrier (BBB) tightly seals circulating blood from CNS tissues. It is a complex composite of diverse cells and basal laminas surrounding a specialized endothelial tube. In its simplest version, the capillary segment of the CNS vascular tree, the central part of the BBB, is formed by a vascular tube composed of endothelial cells tightly sealed by tight junctions. The endothelia further differ from other blood vessels by a relative deficiency in transcellular transport systems3. These properties allow the BBB to keep most circulating blood cells and macromolecules from passing into CNS tissues.
The inner endothelial tube is surrounded by pericytes embedded in an inner basal lamina4. This is followed by a concentric extracellular space, which is again surrounded by a second, parenchymal basal lamina that covers astrocytic end feet extending from the neighboring CNS parenchyma. This outer lamina differs from its central counterpart by its molecular composition, and thus presumably also by its functional properties5.
The immune privileged tissues of the CNS also differ from most other organs in their incomplete lymphatic drainage. In fact, in most organs, a steady stream of interstitial fluid clears subcellular material along with phagocytic antigen presenting cells (APCs) through specialized lymphatic vasculature to the nearest peripheral lymph organs, where immune responses against the imported antigens can be mounted. Such vascular drainage is incompletely developed in the CNS. In rodents, particle tracer studies showed CNS lymphatic drainage restricted to a rudimentary lymphatic flow that runs from the olfactory tissue to the deep cervical lymph node6.
Like most biological systems, the barrier function of the BBB and blood retina barrier (BRB) is not absolute. As will be discussed below, a few immune cells pass through the endothelium and enter the tissue parenchyma. There, however, the new arrivals find a barren, hostile milieu. CNS tissue lacks most of the structures required by T lymphocytes to persist and respond properly. The spectrum of cytokines and chemokines is severely limited in the healthy CNS, and the same is true for cell-bound signaling structures, such as MHC molecules, co-stimulatory factors and cell adhesion molecules.
As has been shown, this particular milieu is stringently controlled by functionally active neurons. In fact, it is known that in conditions of neuronal degeneration, such as Alzheimer's, Parkinson's and Huntington's disease, the local CNS milieu shows signs of inflammatory reactivity. In such tissues, microglia cells, the immune sentinels of the CNS, are activated, cytokines and chemokines are upregulated, and often, but not always, inflammatory infiltrates are formed. Neurodegeneration thus shifts the immunologically barren CNS tissue into a friendlier environment for immune cells and immune reaction.
Experimentally, several set-ups support the neuronal control of CNS immune reactivity. A particularly informative system has been the unilateral axotomy of the rodent facial nerve7. This operation results in degenerative changes in the facial nucleus, the location of the facial motor neuronal cell bodies, which become ensheathed with microglia cells that seem to crop off perisomatic synapses. Local cells produce and release soluble immune mediators, and upregulate MHC class I and class II molecules, as well as sets of co-stimulatory proteins. Perhaps most importantly, immune cells, among them T cells, spontaneously infiltrate the lesioned tissue, whereas the counterlateral, unaffected nucleus remains completely free of any changes.
Immune infiltration of axotomized facial nuclei is particularly marked in animals with incipient EAE8. Very similar changes are noted in rodents with EAE, where temperature trauma-induced lesions of CNS tissues become attractive for autoimmune inflammatory infiltrates, even if placed in regions commonly free of EAE inflammation.
In vitro studies of spontaneously forming neuronal circuits either in explants or dissociated cell cultures revealed that glia cells (microglia as well as astrocytes) near “firing” neurons lacking MHC molecules, although both cell types possess the relevant machinery in the absence of neurons. MHC molecules are also inducible in explants by inactivating local neurons, for example using the sodium channel blocker tetrodotoxin9.
The mechanisms used by neurons to suppress MHC molecules in surrounding glia cells remain unclear, although there is evidence that neurotrophins may be involved. Addition of nerve growth factor, for example, at least partly restores suppression to tetrodotoxin paralyzed neurons10. Another structure that might function in neuronal regulation of the CNS immune milieu is fractalkine, a chemokine present in the CNS both bound to (neuronal) cell surfaces and in a soluble form. Fractalkine acts on glia cells expressing the specific receptor by repressing activating signals, dampening neurotoxic effects by activated microglia11.
The immune privilege of ocular tissues is maintained by most of the structural specializations described above that act in the CNS. For example, the blood vessels form a largely impermeable BRB by interconnecting endothelial cells with dense arrays of tight junctions12. However, the eye uses additional mechanisms to prevent potentially pathogenic inflammatory responses.
In this context, the anterior chamber (AC) of the eye is a particularly immune-privileged site13. Introduction of antigen into the AC triggers a deviant systemic immune response, resulting in the generation of antigen-specific regulatory T cells and suppression of delayed type-hypersensitivity (DTH), rather than an overtly inflammatory response. This phenomenon was initially described as F1 lymphocyte-immune deviation14, 15, 16 and subsequently termed “anterior chamber associated immune deviation” (ACAID)16. The antigenic signal delivered to the AC is carried into the circulation and delivered to the spleen16, 17 where regulatory T cells are generated18. CD-1 reactive natural killer T cells are required for the inhibition of DTH after the AC injection of antigen19. DTH is a form of T cell-mediated immunity that plays an important role in protection against pathogens.
Inhibition of DTH (after intraocular injection) has been reproduced in vitro17, 20, revealing that several immunosuppressive factors are important in the induction of tolerance to antigen21, including complement (C) of the innate immune system22. Suppression of DTH (identical to ACAID) can also be evoked by the intravenous injection of APC pulsed in vitro with soluble antigen in the presence of immunosuppressive factors found in the ocular microenvironment23.
Recent studies found that cells leaving the eye after antigen exposure traffic to the marginal zone (MZ) of the spleen and that two types of APC promote tolerance in the MZ – F4/80+ APC and MZ B cells. Both communicate with iNKT cells via the CD1d molecule expressed on their cell membranes and result in the generation of CD8+ Treg cells24, 25, 26.
Autoantigenic strength
In his Clonal Selection Theory, Burnet postulated that immune cell clones with receptors for self-antigens are deleted from the immune repertoire, thus ensuring immunological self-tolerance. This concept has been substantiated in its basic tenets by an overwhelming body of experimental data. Indeed, the immune system depends on the thymus, a central immune organ, where naïve, diverse T cells are generated, and where most self-reactive clones are removed by various complementary mechanisms. However, at the same time, it has become clear that the deletion machinery is less than perfect. Peripheral T cell populations in both primates and rodents abound with T cells specifically responding to tissue-specific autoantigens, including antigens from the CNS and eye.
Numerous theories have been proposed to explain the failed functions allowing autoreactive T cells to pass through the thymus, and whether autoreactive T cells could have a positive, physiological role in the body. We will not discuss these questions here, but instead consider another, rarely treated aspect of autoimmunity, namely “autoantigenic strength”.
It is long known that most, if not all CNS and eye molecules qualify as potential autoantigens, ie that practically all protein structures may by recognized by a complementary T cell clone preexisting in the healthy immune repertoire. Yet, there are certain tissue specific proteins that stand out due to their particularly high degree of immunogenicity, making them targets for pathogenic T cell responses in autoimmune diseases (Table 1). Furthermore, the linear sequences of such known autoantigens are not all equally autoimmunogenic; the autoantigenic determinants of an autoantigen are commonly restricted to particular, characteristic molecular determinants.
Table 1. Selected rodent and primate EAE (and EAU models) and their indications representing aspects of MS37.
| Strain | Antigen | Induction | Clinical course | Pathology |
|---|---|---|---|---|
| MOUSE-EAE/EAU | ||||
| C57BL6 (H-2b) | Spinal cord homogenate | Active | Resistant | |
| MOG (aa 35–55) | Active, passive | Severe, chronic-progressive, non-remitting | CNS demyelination, acute inflammation, axonal damage | |
| IRBP (aa 1–20) | Active | Mild, acute disease | Ocular inflammation | |
| Passive | Severe disease | |||
| PLP | Passive | Very mild, resistant | ||
| B10RIII | IRBP (aa 161–180) | Active | Severe | Ocular inflammation |
| Passive | Severe | |||
| C3H.SW (H-2b) | MOG (aa 35–55) | Active | Severe, chronic non-remitting | Inflammation, demyelination |
| SJL (H-2s) | Spinal cord homogenate | Active | Relapsing-remitting, Progressive (SCH, MBP) | Demyelination, inflammation |
| MBP | Active, passive | Relapsing-remitting | Demyelination, inflammation | |
| PLP | Active, passive | Relapsing-remitting | Demyelination, inflammation | |
| MOG (aa 92–106) | Active, passive | Severe acute | Inflammation and demyelination | |
| MAG (myelin-associated glycoprotein) (aa 97–112) | ||||
| MOBP (myelin-associated/oligodendrocyte basic protein) (aa 37–60) | Active | Non-relapsing | Inflammation | |
| CNPase (2′,3′-cyclic nucleotide 3′-phosphodiesterase) | ||||
| OSP (oligodendrocyte-specific glycoprotein) | ||||
| (B6 x SJL) F1 (H-2b/s) | MOG | Active | Relapsing-remitting | Inflammation, demyelination |
| B10.S (H-2s) | CNS homogenate | Active | Resistant | |
| MOG | Active | Resistant | ||
| ASW (H-2s) | MOG (aa 92–106) | Active | Depending on Bordetella pertussis application: +secondary progressive (SP)-primary progressive (PP) | Acute inflammation, demyelination |
| Biozzi AB/L (H-2s) | Brain homogenate | Resistant | ||
| Biozzi AB/H (H-2dq1) | Spinal cord homogenate | Active | Relapsing-remitting | Inflammation, demyelination in relapse phase |
| MBP (aa 12–26; 21–35) | Active | Native MBP: resistant,MBP 12–26 and MBP 21–35: mild acute | Inflammation | |
| MOG (aa 1–22; 43–57; 134–148) | Active | Chronic relapsing (MOG 1–22) | Inflammation, no demyelination | |
| Chronic acute (MOG 35–55) | Native MOG: demyelination | |||
| PLP (aa 56–70) | Active | Chronic relapsing | Inflammation, demyelination | |
| Ab-crystallin (aa 1–16) | Active | Acute mild | Inflammation | |
| GFAP (glial fibrillary acidic protein) | Active | Acute severe | Inflammation | |
| ABH (H-2Ag7) | MAG (aa 97–112) | |||
| OSP | ||||
| CNPase | ||||
| NOD/Lt (H-2g7) | rMOG, MOG (aa 35–55) | Active | CREAE | Demyelination with peptide |
| NOD congenic strain III (H-2g7) | MOG | Active | Resistant | |
| BALB/c (H-2d) | Spinal cord homogenate | Active | Resistant | |
| MBP (aa 59–76) | Passive | aEAE resistant, tEAE: acute | Inflammation, demyelination | |
| PLP (aa 178–191) | Active | Relatively resistant, atypical EAE | ||
| B10.PL (H-2u) | MBP (aa Ac1–9) | Active, passive | ||
| PL/J (H-2u) | MBP (aa 89–169) | Active, passive | CREAE | Demyelination, acute inflammation |
| MBP (aa 1–37) | CREAE and chronic persisting paralysis | |||
| MOG (aa 35–55) | Active, passive | Relapsing-remitting | Demyelination, acute inflammation | |
| PLP (aa 43–64) | Active, passive | CREAE | Demyelination, acute inflammation | |
| DBA/1 (H-2q) | Spinal cord homogenate | Active | Resistant | |
| MOG (aa 79–96) | Active | Acute | Inflammation, demyelination | |
| SWR/J (H-2q) | Spinal cord homogenate | Active | ||
| PLP | Active | |||
| C3/HEJ (H-2k) | PLP | Active | Atypical EAE | Inflammation |
| RAT-EAE/EAU | ||||
| Lewis (RT1-l) | Spinal cord homogenate | Active | Acute | CNS inflammation |
| MBP (aa 68–88) | Active and passive | Acute, relapses after cyclosporine | CNS inflammation | |
| PLP | Active and passive | Weak acute | CNS inflammation | |
| MOG (aa 35–55) | Active and passive | Weak acute, after native MOG chronic progressive | CNS inflammation, demyelination with native MOG | |
| S100β (aa 76–91) | Passive | Weak acute | CNS inflammation | |
| GFAP | Passive | Weak acute | CNS inflammation | |
| IRBP (aa 177–1091) | Active | Acute, severe | Ocular inflammation | |
| Passive | Chronic, severe | |||
| S-antigen | Active | Acute, modest | Ocular inflammation | |
| Dark Agouti (DA) (RT1-av1) | Spinal cord homogenate | Active in incomplete FA | CREAE | CNS inflammation and demyelination |
| MBP (aa 62–75) | Active and passive | Acute | CNS inflammation | |
| MOG | Active and passive | Chronic, relapsing-remitting | CNS inflammation and demyelination | |
| BTN (Butyrophilin) (aa 74–90) | Active | No disease | CNS inflammation | |
| Brown Norway (BN) | MBP | Passive | Acute | CNS inflammation |
| (RT1-n) | Rat spinal cord | Active | Acute | CNS inflammation |
| MOG | Active | Chronic progressive | CNS inflammation, demyelination | |
| PVG (RT1-c) | MBP | Active resistant, passive susceptible | Acute | CNS inflammation |
| NON-HUMAN-PRIMATE-EAE | ||||
| Macaca mulatta (Rhesus monkey) | Rabbit brain extract | Active | Acute and chronic progressive | CNS inflammation and demyelination |
| (Mamu-DPB1*01) | ||||
| Brain extract with adjuvant | Active | Acute and chronic | CNS inflammation with necrosis | |
| MOG (aa 4–20, 35–50, 94–116) | Active | Acute and chronic | CNS inflammation with necrosis | |
| MBP (aa 61–82, 80–105, 170–186) | Active and passive | Acute and chronic | CNS inflammation with necrosis | |
| Macaca fascicularis (Cynomolgus monkey) | Brain homogenate | Active | Acute and CREAE | CNS inflammation, necrotic lesions |
| MBP | Active | Acute and CREA | CNS inflammation, necrotic lesions | |
| Macaca nemestrina | Brain homogenate and MBP | Active | Relatively resistant | |
| Common marmoset (Calithrix jacchus) | Myelin homogenate | Active | Acute – chronic progressive | CNS inflammation, demyelination, necrotic lesions |
| Caja-DRB*W1201 | MBP | Active and passive | Mild acute | CNS inflammation, no demyelination |
| PLP | Active | Mild acute | CNS inflammation, no demyelination | |
| MOG (aa 1–124, 14–36) | Active | RR, secondary progressive | CNS inflammation and demyelination | |
There are numerous factors influencing the autoantigenicity of a self-protein, such as availability, processing, binding to MHC class II molecules, affinity of peptide/MHC complexes to TCR, etc, potentially dictating the pathogenic potential of a given self-protein.
Obviously, availability to APCs is a critical feature. Indeed, this is not a trivial prerequisite. Recent studies of APCs isolated from the CNS without severing the parenchyma indicate that the level of local autoantigen presented is remarkably low, too low to trigger T cell proliferation, but sufficient to re-activate numerous pro-inflammatory cytokine and chemokine genes and genes involved in T cell migration27. Addition of exogenous autoantigen by intrathecal infusion of soluble MBP promptly exacerbates a nascent response, but ultimately leads to premature T cell apoptosis via activated cell death28.
Very recently, a phenomenon called cumulative immunity was recognized that could well determine the autoantigenic power of a tissue specific self-antigen. Krishnamoorthy et al studied immune reactivity in a transgenic mouse expressing a MOG-specific T cell receptor on a large proportion of its CD4+ T cells. About 5%–10% of these mice spontaneously develop autoimmune inflammation affecting the optic nerve and spinal cord. Surprisingly, when crossed into a MOG-deficient background, ie, in the absence of the nominal autoantigen, spontaneous EAE was noted with a similar frequency. A detailed proteomic investigation revealed that the MOG-specific TCR co-recognized a particular epitope on the middle neurofilament (NF-M18-30), a component of the axonal cytoskeleton. Transfer studies confirmed that MOG/NF-M double reactive T cells transferred to MOG-deficient mice induced EAE with a delayed onset compared to wildtype, MOG sufficient recipients, whereas double knock-out mice lacking both MOG and NF-M remained completely resistant29.
Is the unexpected cross-reactivity between MOG35-55 and NF-M18-30 the “private” reaction pattern of one particular T cell clone, namely 2D2, or is it commonly found among I-Ab restricted, MOG35-55 reactive T cells? The latter seems to be true. In fact, among a series of MOG35-55, I-Ab restricted T cell clones freshly selected from a primed C57BL/6 mouse, a substantial number, but not all, cross-reacted against NF-M18–30.
The findings might indicate that when recognizing epitopes in the same tissue at the same time, although the epitopes came from distinct protein structures and derived from different cell sources, T cell could add up both response components into a particularly strong cumulative autoimmune response. Obviously, this concept has to be validated in independent autoimmune situations.
Failed immune privilege: Invasion of privileged tissues by autoimmune T cells
Nevertheless, the immune privilege in the CNS and eye is by no means perfect. In fact, there are rare, but spectacular instances where the immune privileged status is abandoned, and lymphocytes and other inflammatory cells seemingly gain free access to the shielded tissues. As expected, such inappropriate inflammation can have devastating consequences. In human MS, for example, the influx of autoimmune T cells responding to CNS autoantigens sets off a pathological chain of reactions that can lead to a patient suffering severe neuronal dysfunction and disability. Similarly, immune privilege of the eye may be lost, with similarly severe consequences, namely loss of vision.
Fortunately, notwithstanding the considerable number of peoples suffering from autoimmune CNS or eye disease, loss of immune privilege in either organ remains a relatively exceptional event. This is explained partly by the complex barrier function of CNS and eye blood vessels, which allows only a very few specialized immune cells to pass into the surrounding tissue.
It has been known for a long time that contrary to traditional concepts of immune reactivity, the healthy immune repertoire is by no means depleted of self-reactive immune cells. In fact, the peripheral blood of healthy people contains an amazing proportion of T cells with receptors recognizing CNS autoantigens30. However, to break through the blood-tissue barrier, an autoimmune T cell has to acquire a very particular set of functional properties. First of all, as demonstrated by transfer experiments in rodents, CNS reactive T cells only transfer EAE to recipients if they had been maximally activated before injection31. Resting autoimmune T cells would not cause disease, even if transferred in enormous numbers, and activation of autoimmune T cells per se is not sufficient to open the way through the blood-tissue barriers. It has been known ever since the first transfers of myelin specific T cell lines that clinical disease and histological lesions do not arise directly after T cell injection, but that clinical EAE develops after a latency period of 3 or more days after transfer. Until recently, it remained unknown where the transferred cells first reside in the recipient's body, and why they enter the CNS only after a protracted period of time.
A study using genetically modified encephalitogenic effector T cells expressing the green fluorescent protein (GFP)32 showed that indeed, the transferred activated T cells travel through the peripheral immune system up to day 3–4 after transfer when they enter the CNS. After initially infiltrating the lung vasculature, the T cells settle in peripheral immune organs, first in perithymic lymph nodes and subsequently in the spleen.
Within the immune milieu, the migrant T cells undergo remarkable changes in their gene expression profile, which prove to be indispensable for enabling the T cells to reach the target organ and invade it. Changes in gene expression are in both directions: A microarray analysis comparing the transcriptomes of in vitro activated effector T cells (the cells transferred to recipient rats) with T cells resident in the spleen (the T cells ready to invade the CNS) identified an estimated 600 down-regulated, and about 300 up-regulated genes in the spleen milieu. The down-regulated genes include numerous T cell activation markers, such as CD25, whereas a series of genes involved in cell migration (chemokines and receptors, etc) were induced33. Intriguingly, KLF-2, a master gene controlling T cell migration and quiescence34 was among the elevated genes, suggesting a regulatory role for this gene. Interestingly, KLF-2 controls the transcription of sphingosin-1-phosphate receptors, which are not only centrally involved in regulating T cell egress from lymphoid organs, but have also become promising targets in immunomodulatory therapies for human autoimmunity35. In fact, although without experimental proof so far, the KLF-2/sphingosin-posphate receptor axis may contribute to the signals triggering the mass exodus of reshaped migratory effector T cells from the spleen 3–4 days after transfer.
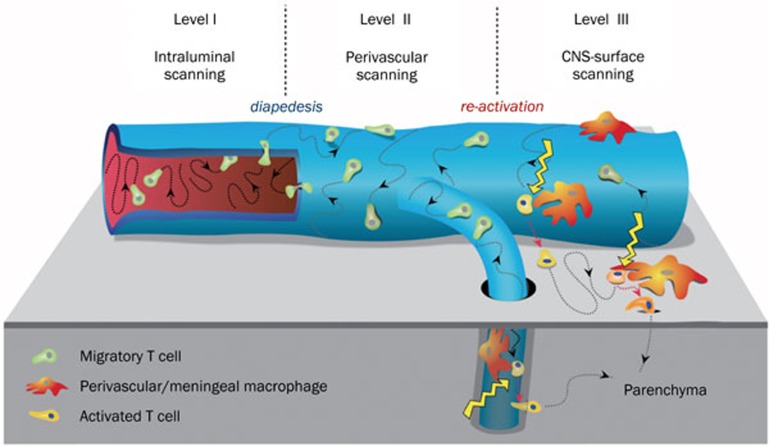
Upon reaching the CNS blood vessels, the migratory T cells face a highly sophisticated barrier system. Indeed, as shown by recent real-time imaging studies using two-photon microscopy, the T cell use extremely elaborated strategies to overcome this barrier27. Freshly arriving T cells attach to the luminal surface of BBB vessels, and instead of immediately crossing the vascular wall, they first crawl over the surface, preferentially against the blood flow. Actual extravasation occurs only after an extensive period of scanning. On the abluminal side of the BBB vessel, the T cells continue their crawling locomotion until they contact one or more of the local phagocytes surrounding the blood vessel. Although these phagocytes are distinct from classical macrophages and dendritic cells, they are able to productively present antigen to T cells. In particular, the BBB associated phagocytes present locally produced autoantigens, although apparently at low doses. Autoantigen recognition is sufficient to partially activate incoming encephalitogenic T cells by inducing a set of immune mediators and their receptors, but does not trigger full activation with cell proliferation. Rather, serial contacts of encephalitogenic effector T cells with local autoantigen-presenting phagocytes appear to guide the T cells to the surface of the CNS and ultimately into the parenchyma (Figure 1).
Figure 1.
Three-level interaction of migratory autoimmune T cells with the blood-brain barrier. Blood-borne T cells attach to the inner surface of a CNS associated blood vessel, where they crawl randomly, but mainly against the blood stream (Level I). The T cells cross the vessel wall and continue crawling on the outer surface (Level II), until they make contact with local perivascular phagocytes. These phagocytes present local autoantigens and signal the migratory T cells to enter the CNS parenchyma (Level III). [Adapted from 27]
Within the CNS white matter, myelin specific effector T cells initially migrate through the dense parenchyma at remarkably high speed. They are stopped by local antigen presenting cells, either activated microglia or intruded peripheral macrophages, which express high levels of MHC class II proteins. The effector T cells dock onto these cells and form immune synapses, juxtaposing T cell receptor-enriched membrane areas with MHC class II containing membranes of the antigen presenting cells. As a result of these contacts, the T cells become highly activated, producing high levels of pro-inflammatory cytokines and chemokines36. Either directly or indirectly via recruitment and activation of accessory cells, this activation process is responsible for the functional and structural changes underlying clinical EAE.
Implications
Immune privilege has evolved particularly elaborate structural organization in certain tissues, such as the CNS or eye, in order to avoid unacceptable collateral damage connected with inflammatory responses routinely triggered by immune surveillance. However, the immune privilege of the CNS and eye does not mean complete lack of protection. First, elements of innate immune responsiveness provide some defense; and second, a specialized subset of T cells is able to overcome the vascular barriers to enter the shielded tissue. These cells have a beneficial function in screening the CNS and eye for microbial agents, but they are pathogenic if they respond to local self-antigens. Detailed insights into the mechanisms of tissue invasion by autoimmune migrant T cells could provide a basis for specific treatments of autoimmune diseases in immune privileged organs.
References
- Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Salinas-Carmona MC, Nussenblatt RB, Gery I. Experimental autoimmune uveitis in the athymic nude rat. Eur J Immunol. 1982;12:480–4. doi: 10.1002/eji.1830120606. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P. Brain endothelial cells and the glio-vascular complex. Cell Tiss Res. 2009;335:75–96. doi: 10.1007/s00441-008-0658-9. [DOI] [PubMed] [Google Scholar]
- Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–78. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- Korpos E, Wu C, Song J, Hallmann R, Sorokin L. Role of the extracellular matrix in lymphocyte migration. Cell Tiss Res. 2010;339:47–57. doi: 10.1007/s00441-009-0853-3. [DOI] [PubMed] [Google Scholar]
- Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of the rat brain. Am J Physiol. 1984;246:F835–44. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CUA, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Maehlen J, Olsson T, Zachau A, Klareskog L. Local enhancement of major histocompatibility complex (MHC) class I and class II expression and cell infiltration in experimental allergic encephalomyelitis around axotomized motor neurons. J Neuroimmunol. 1989;23:125–32. doi: 10.1016/0165-5728(89)90031-3. [DOI] [PubMed] [Google Scholar]
- Neumann H, Boucraut J, Hahnel C, Misgeld T, Wekerle H. Neuronal control of MHC class II inducibility in rat astrocytes and microglia. Eur J Neurosci. 1996;8:2582–90. doi: 10.1111/j.1460-9568.1996.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Neumann H, Misgeld T, Matsumuro K, Wekerle H. Neurotrophins inhibit major histocompatibility class II inducibility of microglia: Involvement of the p75 neurotrophin receptor. Proc Natl Acad Sci USA. 1998;95:5779–84. doi: 10.1073/pnas.95.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Crane IJ, Liversidge J. Mechanisms of leukocyte migration across the blood-retina barrier. Semin Immunopathol. 2008;30:165–77. doi: 10.1007/s00281-008-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW. Immunologic privilege of the eye. Springer Semin Immunopathol. 1999;21:95–111. doi: 10.1007/BF00810243. [DOI] [PubMed] [Google Scholar]
- Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977;118:809–14. [PubMed] [Google Scholar]
- Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. II. An analysis of F1lymphocyteinduced immune deviation. J Immunol. 1978;120:689–93. [PubMed] [Google Scholar]
- Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med. 1981;153:1058–67. doi: 10.1084/jem.153.5.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID depends upon intraocular transforming growth factor-beta. Eur J Immunol. 1992;22:165–73. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990;71:383–9. [PMC free article] [PubMed] [Google Scholar]
- Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–26. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orazio TJ, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the induction of anterior chamber-associated immune deviation (ACAID) Immunology. 1998;95:47–55. doi: 10.1046/j.1365-2567.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Curr Opin Immunol. 1993;5:428–32. doi: 10.1016/0952-7915(93)90064-y. [DOI] [PubMed] [Google Scholar]
- Sohn JH, Bora PS, Suk HJ, Molina H, Kaplan HJ, Bora NS. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Nat Med. 2003;9:206–12. doi: 10.1038/nm814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Okamoto S, Rouse B, Streilein JW. Evidence that peritoneal exudate cells cultured with eye-derived fluids are the proximate antigen-presenting cells in immune deviation of the ocular type. J Immunol. 1993;151:5162–71. [PubMed] [Google Scholar]
- Nakamura T, Sonoda KH, Faunce DE, Gumperz J, Yamamura T, Miyake S, et al. CD4+ NKT cells, but not conventional CD4+ T cells, are required to generate efferent CD8+ T regulatory cells following antigen inoculation in an iImmune-privileged site. J Immunol. 2003;171:1266–71. doi: 10.4049/jimmunol.171.3.1266. [DOI] [PubMed] [Google Scholar]
- Faunce DE, Sonoda KH, Stein-Streilein J. MIP-2 recruits NKT cells to the spleen during tolerance induction. J Immunol. 2001;166:313–21. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- Lin HH, Faunce DE, Stacey M, Terajewicz A, Nakamura T, Zhang-Hoover J, et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J Exp Med. 2005;201:1615–25. doi: 10.1084/jem.20042307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- Odoardi F, Kawakami N, Klinkert WEF, Wekerle H, Flügel A. Blood-borne soluble protein antigen intensifies T cell activation in autoimmune CNS lesions and exacerbates clinical disease. Proc Natl Acad Sci USA. 2007;104:18625–30. doi: 10.1073/pnas.0705033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy G, Saxena A, Mars LT, Domingues HS, Mentele R, Ben-Nun A, et al. Myelin specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nat Med. 2009;15:626–32. doi: 10.1038/nm.1975. [DOI] [PubMed] [Google Scholar]
- Pette M, Fujita K, Wilkinson D, Altmann DM, Trowsdale J, Giegerich G, et al. Myelin autoreactivity in multiple sclerosis: Recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple sclerosis patients and healthy donors. Proc Natl Acad Sci USA. 1990;87:7968–72. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–9. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- Flügel A, Willem M, Berkowicz T, Wekerle H. Gene transfer into CD4+ T lymphocytes: Green fluorescent protein engineered, encephalitogenic T cells used to illuminate immune responses in the brain. Nat Med. 1999;5:843–7. doi: 10.1038/10567. [DOI] [PubMed] [Google Scholar]
- Flügel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, et al. Migratory activity and functional changes of green fluorescent effector T cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–60. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;422:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–63. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N, Nägerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flügel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–14. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H, Kurschus FC. Animal models of multiple sclerosis. Drug Discov Today Dis Models. 2006;3:359–67. [Google Scholar]