IgE antibodies are involved not only in the immune defense against parasites, but also in the pathogenesis of allergic diseases and anaphylaxis. Allergen-induced crosslinking of IgE bound to FcεR1 on mast cells triggers degranulation and may initiate an allergic reaction.1 Although IgE is a major player in allergic responses, IgE regulation and the molecular pathway underlying IgE+ B-cell maturation remains elusive. In an exciting report published in the April issue of Nature Immunology, Talay and colleagues demonstrate a new model for IgE switching and memory in vivo.2 In contrast to the current paradigm, IgE+ memory B cells and plasma cells develop through a germinal-center (GC) IgE+ intermediate, without the need of a transitional IgG1 phase. Furthermore, IgE+ memory B cells, and not IgG1+ memory B cells, are the source of cellular IgE memory.
The typical immune response to T cell-dependent antigens starts with B-cell differentiation into short-lived plasma cells that produce low-affinity antibodies, followed by the formation of GCs that are the origin of both memory B cells and long-lived plasma cells that produce high-affinity antibodies.3 Previous studies reported a pathway of IgE B-cell maturation that is distinct from the classical model of B-cell differentiation.4,5 IgE+ B cells were not found in GCs nevertheless differentiated into high-affinity plasma cells. It was proposed that GC IgG1+ intermediates switched to IgE+ plasma cells outside the GCs.4 This model was strengthened by the observation that IgG1-deficient mice were impaired in producing high-affinity IgE.5 Moreover, cellular IgE memory was reported to reside in IgG1+ memory B cells which undergo a sequential switch to IgE after re-exposure to antigen.4
IgE differentiation is difficult to study since IgE-expressing B cells are very rare under physiological conditions and the detection of IgE+ cells by antibody staining is complex as cells expressing Fcε receptors can bind IgE.1 The previously described detection method, which consists of acid treatment to remove serum IgE bound to CD23, followed by staining with anti-IgE, may lack the sensitivity needed to detect the low surface expression of IgE on IgE+ GC B cells.
In the current study by Talay and colleagues, this problem is circumvented by the use of sensitive IgE reporter mice. Here, a bicistronic reporter gene encoding-enhanced green fluorescent protein (GFP) is inserted into the murine locus encoding IgE downstream of the cytoplasmic domain of membrane IgE. This elegant method allows for the specific and direct detection of both IgE-switched B cells and plasma cells. After validation of the reporter, they investigate IgE switching and memory using two models: infection of mice with the helminth Nocardia brasiliensis and a classical immunization with TNP-ovalbumin, which both result in a strong T helper 2 response associated with specific IgG1 and IgE production.
IgE-switched cells, identified as GFPhi B cells, are found in lymph nodes (mediastinal and mesenteric) and spleen 7–10 days after infection. Talay et al. demonstrate that almost 100% of the GFPhi cells display a GC B-cell phenotype (B220+IgD−GL7+CD95+) at day 10, which decreases to 60%–80% of GFPhi cells at day 35 after infection. Additionally, a small population of IgE+ memory B cells (B220+IgD−GL7−CD38hi) is detected. These results are in contrast to previous studies in which IgE+ cells were not found in GCs and had plasma cell characteristics. Talay and colleagues reveal that 15%–20% of all plasma cells (B220−CD138+) in the lymph node and 3% of plasma cells in the spleen are IgE+ 15 days after infection. Remarkably, in the bone marrow only 1% of all plasma cells are IgE+ at day 35, which suggests that IgE+ plasma cells are rather short-lived. Their findings are strengthened by confirming the results in the TNP-ovalbumin model. In line with the flow cytometry data, the authors find GFP+ cells in IgD−GL7+ GC structures in lymph nodes of infected mice by immunofluorescence microscopy. IgE+CD138+ plasma cells are located distant from GCs and IgE+ memory B cells are found in clusters next to GL7+ GCs.
To validate that cellular IgE memory resides within IgE+ memory cells and to rule out a role for IgG1+ memory cells, adoptive transfer experiments were conducted. GFPhiCD38hi memory B cells were sorted from the reporter mice and transferred into B cell-deficient mice. They demonstrate a potent IgE serum response in the recipient mice starting 5 days after infection, with similar kinetics as a secondary infection in intact IgE reporter mice. Importantly, in mice that received sorted B220+IgD−GL7−CD38hiGFPint-neg non-IgE memory cells, no IgE serum response is generated after infection with N. brasiliensis. Therefore, a putative role for IgG1+ memory cells as the source of IgE memory can be excluded.
A recent study by Yang et al., using different IgE reporter mice, confirms that IgE+ B cells differentiate into GC B cells.6 However, the presence of IgE+ B cells in the GC is transient, and IgE+ plasma cells show reduced affinity maturation compared to IgG1+ plasma cells. They conclude that IgE+ B cells show a bias toward differentiation into short-lived plasma cells. This fits with the study by Talay et al. considering the kinetics of serum IgE and the very low number of IgE+ plasma cells found in the bone marrow. The skewed differentiation into short-lived plasma cells may provide a mechanism to prevent aberrant IgE antibody production and systemic anaphylaxis. The new model of IgE production by Talay and colleagues provides us with a deeper understanding of IgE biology (Figure 1), and may reveal new opportunities for the prevention of allergic responses.
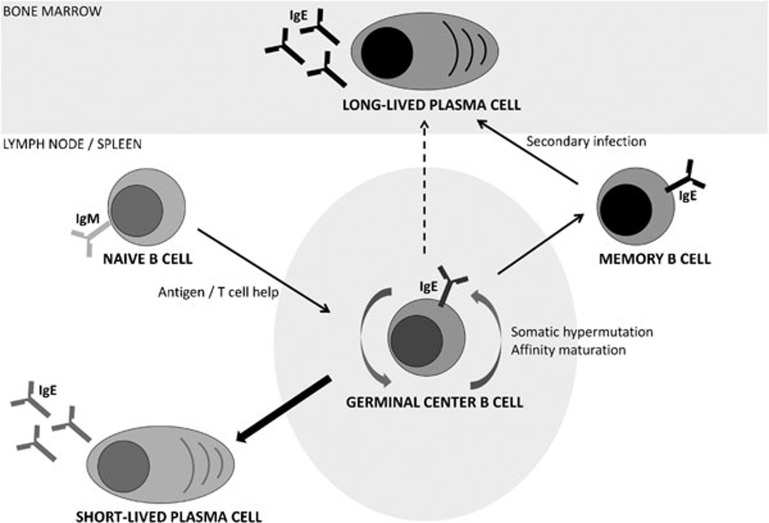
Figure 1.
A new model for IgE plasma-cell and memory B-cell differentiation. After T cell-dependent activation, naive IgM+ B cells switch to IgE and enter the germinal-center reaction. Germinal-center IgE+ B cells (B220+IgD−GL7+CD95+) undergo somatic hypermutation and affinity maturation while differentiating into short-lived IgE+ plasma cells (B220−CD138+) (thick arrow). A very small population of high-affinity long-lived plasma cells migrates to the bone marrow (dashed line). In addition, high-affinity IgE+ memory B cells (B220+IgD−GL7−CD38hi) are generated, which are the source of cellular IgE memory and can differentiate into IgE+ plasma cells upon re-encounter of antigen.
References
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, et al. IgE memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. 2012;13:396–404. doi: 10.1038/ni.2256. [DOI] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Erazo A, Kutchukhidze N, Leung M, Christ AP, Urban JF, Jr, Curotto de Lafaille MA, et al. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sullivan BM, Allen CD. Fluorescent in vivo detection reveals that IgE+ B cells are restrained by an intrinsic cell fate predisposition. Immunity. 2012;36:1–16. doi: 10.1016/j.immuni.2012.02.009. [DOI] [PubMed] [Google Scholar]