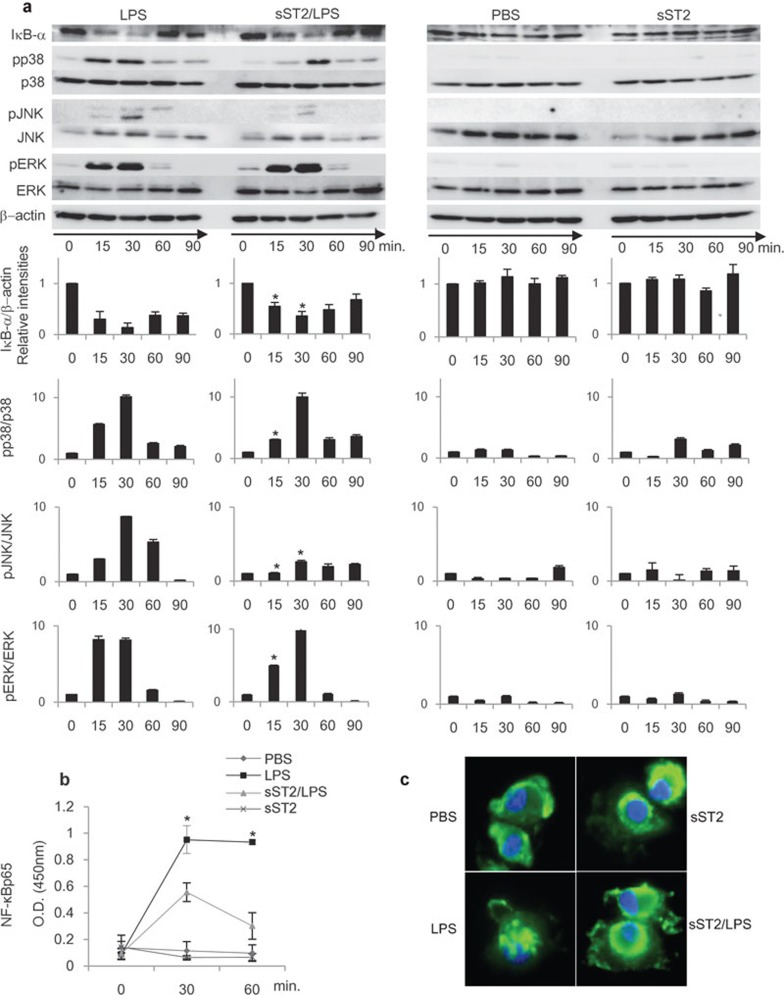
Figure 3.
LPS signaling pathway was attenuated by sST2. (a) sST2 inhibited IκBα degradation and phosphorylation of MAPKs. iMDDCs with or without sST2 pre-treatment were stimulated by LPS (500 ng/ml) for 15, 30, 60, or 90 min, and the cell lysates were resolved by SDS–PAGE and transferred to PVDF membranes. The amounts of phosphorylated, total MAPKs, or IκBα were detected using specific antibodies. The relative intensities of the bands were calculated by LAS4000 and normalized with the values at 0 min. β-actin, p38, JNK, or ERK levels were used as control for equal loading. The representative result of three experiments is presented. Data indicated mean values±s.d. An asterisk denotes a statistically significant difference (*P<0.05). (b) Nuclear extracts were separated from iMDDCs with the same stimulus and time course for 15, 30, or 60 min as in (a), and then the amounts of NF-κBp65 in nuclear extracts were analyzed with NF-κBp65 transcription factor assay kit. The data are presented as means of three experiments±s.d. An asterisk denotes a statistically significant difference (*P<0.05). (c) sST2 inhibited nuclear translocation of NF-κBp65. iMDDCs were treated as indicated for 60 min, then incubated with anti-NF-κB p65 antibody for 60 min at room temperature. Representative data of three experiments are presented. iMDDCs, immature monocyte-derived dendritic cell; LPS, lipopolysaccharide; MAPK, MAP kinase; PVDF, polyvinylidene fluoride; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; sST2, soluble ST2 protein.