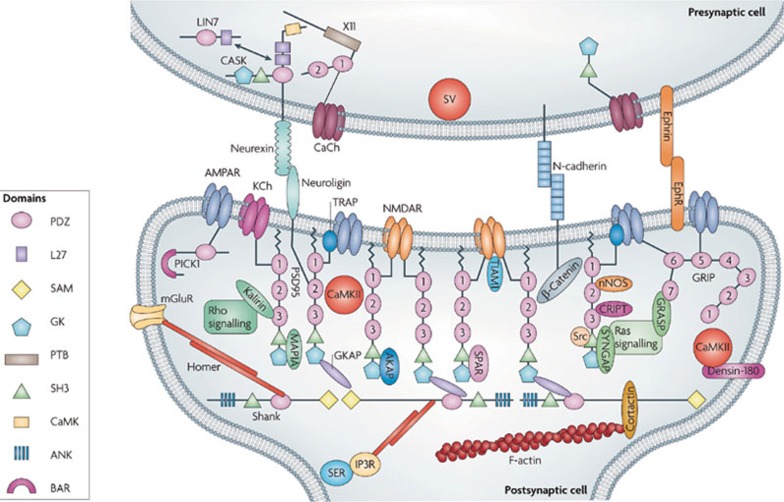
Figure 1.
Model postsynaptic scaffold at a glutamate spine synapse. The postsynaptic density is comprised of membrane receptors and ion channels, scaffold and adaptor proteins, signaling proteins, cell-adhesion molecules and components of the cytoskeleton. Glutamate receptors, such as NMDARs (N-methyl-D-aspartate receptors) and AMPARs (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors), are located in the postsynaptic membrane, with the NMDARs at the center of the synapse and the AMPARs more peripheral. The PDZ-domain-containing scaffold proteins PSD95 (also known as DLG4) and the Src-homology domain 3 (SH3) and multiple ankyrin repeat domains (Shank) family form a two-layer protein network below the postsynaptic membrane, which is bridged by guanylate kinase-associated protein (GKAP). PSD95 forms membrane-perpendicular and roughly equally spaced filamentous structures, with its amino terminus attached to the membrane. Other signaling molecules occupy the spaces in the PSD95–GKAP–Shank protein web. Shank-family scaffolds are further linked to actin filaments. The domains of PSD95 and Shank [PDZ, SH3, guanylate kinase (GK), sterile-alpha motif (SAM) and ankyrin repeats (ANK) (see key)] are shown; other proteins are represented by simple shapes and are labeled. The presynaptic and postsynaptic membranes are connected by cell-adhesion molecules. Reprinted by permission from Macmillan Publishers Ltd: Nat Rev Neurosci 10(2), Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density, 87-99, copyright 2009.