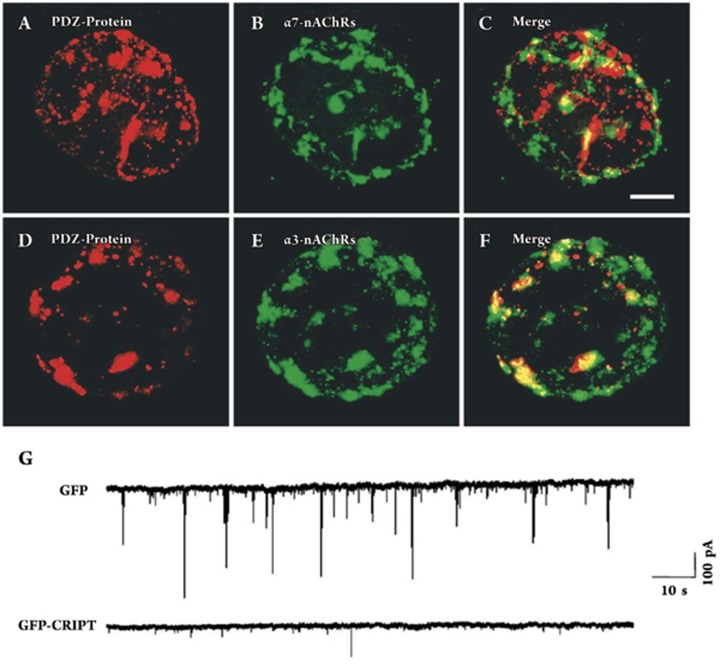
Figure 2.
PDZ proteins codistribute with CG synaptic nAChRs; disrupting PDZ interactions in postsynaptic cells diminishes synaptic transmission. (A) Freshly dissociated E15 CG neurons were immunostained with a monoclonal antibody (mAb) that recognizes the PSD-95 family of PDZ proteins and costained with either goat polyclonal antisera for α7-nAChRs (B) or with mAb 35 for α3β4*-nAChRs (E), and the paired images merged (C, F). Both α7-nAChR and α3β4*-nAChR clusters colocalize with PDZ. Scale bar: 10 μm. (G) CG neurons in culture for 7 days develop α3β4*-nAChR clusters that colocalize with PDZ proteins. CG neurons were transfected on day 1 in culture with GFP-CRIPT, which disrupts all PSD-95 family PDZ interactions. Whole-cell patch-clamp recording revealed many spontaneous EPSCs in control cells transfected with GFP (upper trace) but only relatively few EPSCs in cells transfected with GFP-CRIPT (bottom trace). Reprinted from Neuron, 38(5), Conroy WG, Liu Z, Nai Q, Coggan JS, Berg DK, PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons, 759-71, 2003, with permission from Elsevier.