Abstract
One new order, one new family, and one new combination are presented, as the result of molecular phylogenetic analyses. The new order Stereopsidales and the new family Stereopsidaceae are described incorporating Stereopsis radicans and S. globosa, formerly Clavulicium globosum. We show that not only do these species represent an old overlooked lineage, but both species harbor cryptic diversity. In addition, a third species, C. macounii, appears as a plausible sister to the new lineage, but there is conflict in the data. All specimens of S. radicans and S. globosa analysed here are from the South and Central Americas; several records of S. radicans have been made also from tropical Asia. We expect the true diversity in this group to be a lot higher than presented in this paper. Stereopsis radicans was formerly included in Polyporales, but a placement within that order is rejected by our data through SH tests. The dataset consisted of four nuclear markers: rpb2, tef1, LSU and SSU, each of which was analysed separately using maximum likelihood and Bayesian inference. Recombination detection tests indicate no plausible recombinations. The potential of S. radicans, S. globosa and C. macounii being amphitallic is briefly discussed.
Introduction
Agaricomycetes Dowell, commonly recognized as the mushroom forming fungi, are basidiomycetous fungi forming complex fruiting bodies above and below ground. At present, the Agaricomycetes includes 19 orders [1], [2] dominated by lineages with agaricoid, corticioid, boletoid, polyporoid, and gasteroid sporocarps [3]–[6]. The presence of another unrecognized order within Agaricomycetes was indicated in a recent study of stipitate stereoid fungi, based on nuclear ribosomal 5.8 S and 25 S (LSU) data [7]. Most stipitate stereoid taxa could at that time be assigned to either of the orders Polyporales Gum., Hymenochaetales Oberw., Atheliales Jlich, or Agaricales Underw. However, Stereopis radicans (Berk.) D. A. Reid, the type of Stereopsis D.A. Reid, together with Clavulicium globosum Hjortstam & Ryvarden formed a sister clade to the Cantharellales Gum., the Phallomycetidae Hosaka, Castellano & Spatafora, and remaining orders of Agaricomycetes. Clavulicium macounii (Burt) Parmasto, the type species of Clavulicium Boidin, also did not appear as a member of any of the recognized orders, but separate from the S. radicans - C. globosum clade.
Here we investigate the robustness of the Stereopsis radicans - Clavulicium globosum clade and the placement of C. macounii by analysing the nuclear markers RNA polymerase II subunit (rpb2), translation elongation factor 1 (tef1), and nuclear small subunit ribosomal (SSU) DNA as well as LSU. In addition to phylogenetic analyses, the strength of the Maximum Likelihood trees are tested against alternative trees that would necessitate new taxonomic combinations or that would support existing morphological classifications. The potential of the study group being of a hybrid origin or having received lateral gene transfer leading to disparate phylogenetic signals is investigated through recombination detection tests. These tests would also reveal if there were any chimeric sequences. Gene tree incongruencies are common in the studies of plants and animals [8], [9], but less well investigated in fungal phylogenetics. We are open to the possibility that the genetic markers studied here have different evolutionary histories, and therefore choose to analyse each gene separately.
(tef1), and nuclear small subunit ribosomal (SSU) DNA as well as LSU. In addition to phylogenetic analyses, the strength of the Maximum Likelihood trees are tested against alternative trees that would necessitate new taxonomic combinations or that would support existing morphological classifications. The potential of the study group being of a hybrid origin or having received lateral gene transfer leading to disparate phylogenetic signals is investigated through recombination detection tests. These tests would also reveal if there were any chimeric sequences. Gene tree incongruencies are common in the studies of plants and animals [8], [9], but less well investigated in fungal phylogenetics. We are open to the possibility that the genetic markers studied here have different evolutionary histories, and therefore choose to analyse each gene separately.
We show that a new order is required to adequately summarize the unique evolutionary history represented by these fungi. We also delimit the group of study in relation to the current classifications of Hibbett et al. and Binder et al. [1], [2], and in doing so, show that this newly described order has a previously unrecognized ancient history stretching back 237–290 million years based on comparisons with dated phylogenies of the Agaricomycetes [10].
Results
Phylogenetic analyses
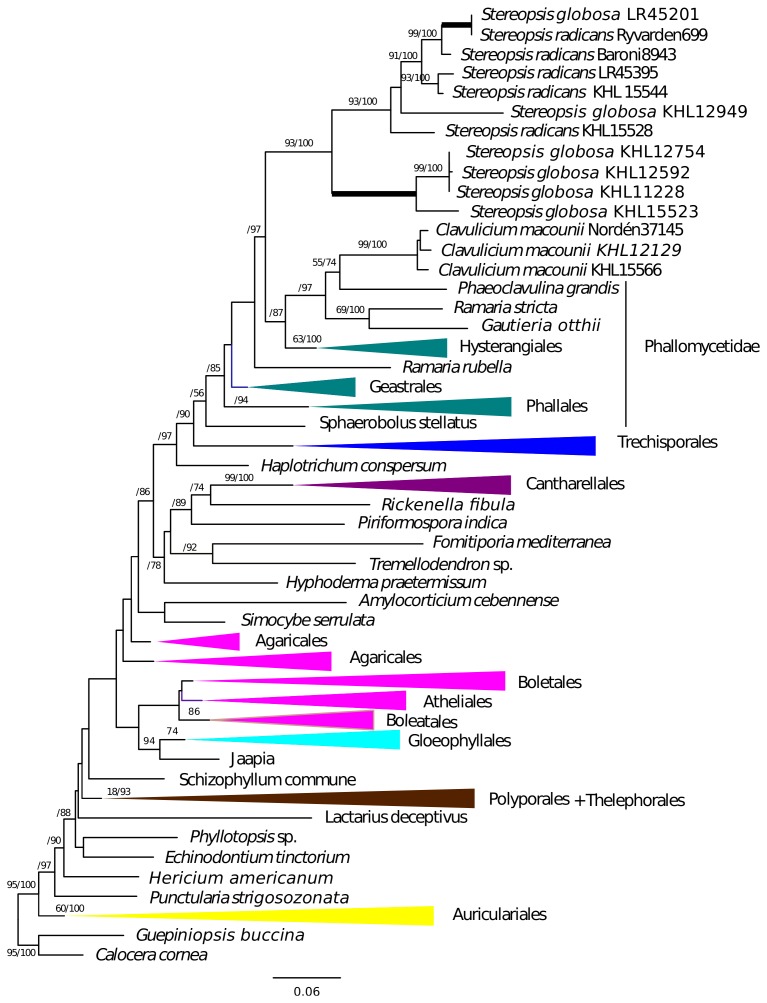
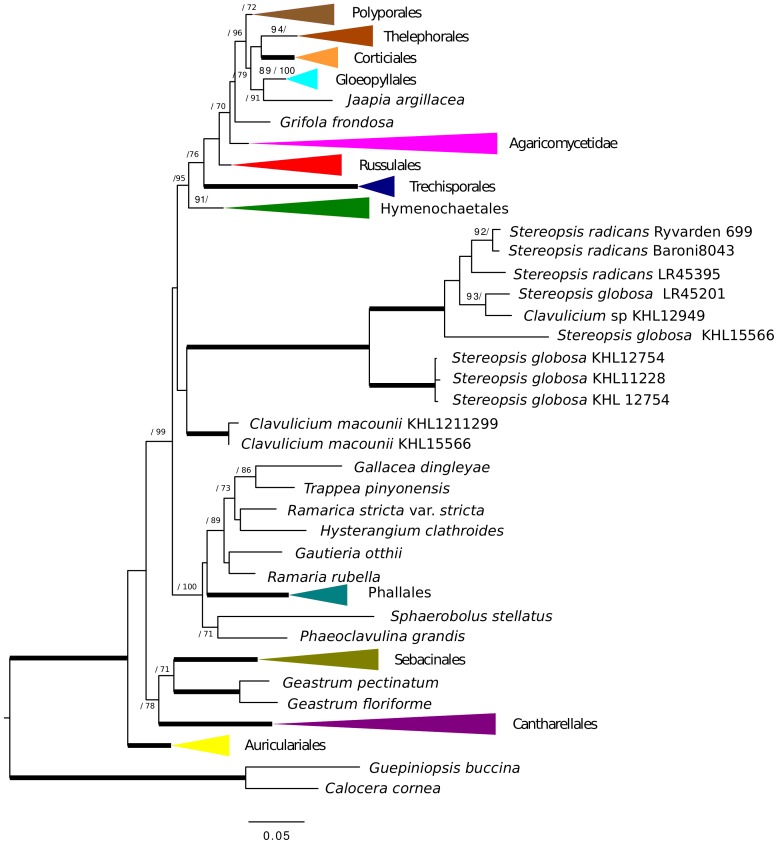
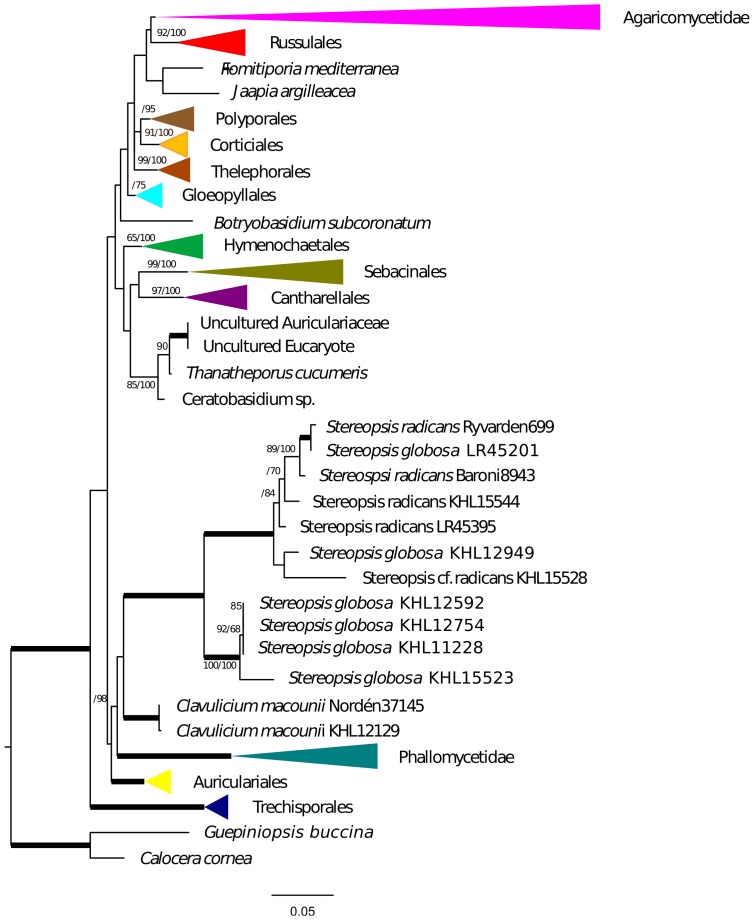
Gene tree analyses of tef1, rpb2, SSU and LSU all reveal the same relationships with regards to Stereopsis globosa and S. radicans, namely that these species form a clade of their own in each tree. The branches supporting the monophyly of S. radicans and C. globosum received 93% bootstrap or higher and a Bayesian posterior probability of 1.0 (Figs. 1, 2, 3, 4). All trees are available from http://purl.org/phylo/treebase/phylows/study/TB2:S14833 for download. In contrast, the placement of C. macounii is not equally clear. Samples from this species appear as the sister group to the S. radicans - C. globosum clade in analyses of rpb2, SSU and LSU, but with bootstrap supports only up to 63% and posterior probabilities between 0.53 and 0.97. Clavulicium macounii was not found to be sister to S. radicans - C. globosum in analyses of tef1. Analyses of rpb2 and SSU reveal a sister relationship between Phallomycetidae and the S. radicans – C. globosum – C. macounii clade, whereas the phylogeny of tef1 shows C. macounii, and the S. radicans - C. globosum clade as a part of a paraphyletic Phallomycetidae. The gene tree of LSU shows the Phallales as polyphyletic, but not as sister to the S. radicans – C. globosum – C. macounii clade.
Figure 1. Rpb2 phylogeny.
Maximum likelihood tree of rpb2 with Bootstrap/Bayesian frequencies as a percentage shown above branches. Thick branches receive full support of both Bayesian frequencies and Bootstrap. The collapsed and colored groups represent current orders and subclasses of the Agaricomycetes.
Figure 2. Tef1 phylogeny.
Maximum likelihood tree of tef1 with Bootstrap/Bayesian frequencies as a percentage shown above branches. Thick branches receive full support of both Bayesian frequencies and Bootstrap. The collapsed and colored groups represent current orders and subclasses of the Agaricomycetes.
Figure 3. LSU phylogeny.
Maximum likelihood tree of nLSU with Bootstrap/Bayesian frequencies as a percentage shown above branches. Thick branches receive full support of both Bayesian frequencies and Bootstrap. The collapsed and colored groups represent current orders and subclasses of the Agaricomycetes.
Figure 4. SSU phylogeny.
Maximum likelihood tree of nSSU with Bootstrap/Bayesian frequencies as a percentage shown above branches. Thick branches receive full support of both Bayesian frequencies and Bootstrap. The collapsed and colored groups represent current orders and subclasses of the Agaricomycetes.
The Stereopsis radicans - S. globosa clade split into two well separated lineages, one containing sequences only from S. globosa specimens, whereas the other contains sequences from both S. globosa and S. radicans specimens. Three specimens of S. globosa have almost identical sequences for several markers, but the remaining specimens appear to be clearly differentiated (Figs. 1, 2, 3, 4).
Additional analyses
We tested whether alternative groupings could be rejected by our data using Shimodaira-Hasegawa (SH) topology tests [11]. The SH tests of monophyly constraints rejected a monophyletic group consisting of Polyporales Gum., Stereopsis radicans, Clavulicium globosum and C. macounii for all markers (Table 1). A monophyletic clade consisting of C. globosum, C. macounii and S. radicans was present in three of the markers, and therefore not available to be tested as an alternative. However, in tef1, this alternative grouping was available and was rejected by the SH test (Table 1). The monophyly of C. macounii with the Phallomycetidae could not be ruled out by the SH tests of SSU, LSU and rpb2, but was rejected in the analyses of tef1. Analyses of LSU recovered S. radicans and C. globosum as sister to C. macounii, but this clade was not recovered as sister to the Phallomycetidae. However, the monophyly constraint of S. radicans, C. globosum and C. macounii together with the Phallomycetidae could not be rejected in the SH test performed on the LSU.
Table 1. Results of the SH tests.
| Marker | ||||
| Hypothesis | rpb2 | tef1 | SSU | LSU |
| H0 | −62365.826866 | −21287.959280 | −16991.358317 | −19069.923892 |
| H1 | - | Yes/−21505.315272 | - | - |
| H2 | - | Yes/−21510.178188 | - | No/−19078.565209 |
| H3 | - | Yes/−21516.149702 | - | No/−19079.216286 |
| H4 | No/−62373.423522 | Yes/−21513.383437 | No/−17019.475756 | No/−19082.748004 |
| H5 | Yes/−62509.372069 | Yes/−21427.239262 | Yes/−17128.704432 | Yes/−19210.024808 |
The question of whether the tested hypothesis results in a significantly worse tree than the ML tree under a p value of 0.05 is answered by a yes or a no. Log Likelihood values for the best tree under each hypothesis is given as a negative value. H1: Stereopsis radicans, C. macounii, C. globosum monophyletic. H2: Phallomycetidae, Stereopsis radicans, C. macounii, C. globosum monophyletic. H3: Phallomycetidae monophyletic and Stereopsis radicans, C. macounii, C. globosum monophyletic. H4: Phallomycetidae, C. macounii monophyletic H5: Polyporales, Stereopsidales monophyletic.
The best matches using blastn [12] searches with Stereopsis radicans and Clavulicium macounii as queries were inferred in all cases (except one) to be members of clades corresponding to the current orders. None of the best blastn matches appear as sister to any of the specimens of study in any of the gene trees.
The structure model analysis of SSU resulted in a similar tree to the regular analysis, and is therefore not shown.
Evidence for recombination events was assessed in RDP4, using the Geneconv, Chimaera, MaxChi, Secondary Bootscan and Secondary SiScan methods [13]–[17]. Detected recombination events were rechecked with all methods, but none of the detected recombinations appeared to be phylogenetically sound. None of the species of interest were recovered as recombinants when using a p-value cut off of 0.05.
Discussion
Stereopsis radicans and Clavulicium globosum form a well supported clade, in all analyses performed here. This result is in concordance with previous analyses [7] based on LSU only. SH tests and ML trees refute the position of the Stereopsis lineage in Polyporales [18]. Clavulicium macounii appears to be sister to the Stereopsis clade, but this position is weakly supported, and rejected by the SH test in one marker. With the relatively short branch lengths supporting the sister relationship of the S. radicans - C. globosum clade and C. macounii, either position of C. macounii is plausible. The position of the Stereopsis lineage is separate from all currently recognized orders of Agaricomycetes Dowell, but appears as a sister lineage to C. macounii and the Phallomycetidae K. Hosaka, Castellano & Spatafora, this relationship is found in three markers. We do not have sufficient data to examine the rank of Phallomycetidae K. Hosaka, Castellano & Spatafora, a position within the subclass is possible but not convincing. A placement within any of the orders of Phallomycetidae K. Hosaka, Castellano & Spatafora is therefore also rejected.
We deem it necessary to describe a new order and a new family to incorporate the newly identified lineage. In doing so, we hope to draw more attention to this lineage which we believe is greatly under-studied, and we hope more records of the species will be reported. Corticioid species are not as well studied as other groups of Agaricomycetes, and the tropical countries, where this lineage is found, are still poorly sampled [19]. The alternative, to not make a formal classification, but instead introduce a temporary informal name, is less compelling. The lineage appears to be at least as robust as other higher ranked taxa in the Agaricomycetes. Our analyses also make it clear that it does not belong to any of the current orders. There is always uncertainty about how to demarcate ranked taxa, since there is no definitive delimiter. However, the ranks should reflect the unique history and predict the distinctive genetic diversity of a group. Therefore, increased knowledge of hitherto unrecognized ancient lineages and the naming and classification of such lineages into higher ranked taxa facilitates communication about them. Most importantly, such classifications also constitute highly important biodiversity information that goes beyond simply the number of species in a given place as the sole criterion for conservation.
Taxonomy
Stereopsis radicans is the type of genus Stereopsis, and Clavulicium macounii the type of Clavulicium. We therefore deem it necessary to transfer C. globosum to Stereopsis, as S. radicans and C. globosum form a monophyletic clade and the monophyly with C. macounii is dubious. This would have no taxonomical impact should C. macounii later be proven sister to the Stereopis clade, so long as S. radicans and C. globosum remain monophyletic. However, we do recognize the possibility that there are several cryptic species that fit the description of the new combination, as well as within S. radicans.
Stereopsis globosa (Hjortstam & Ryvarden) Sjkvist comb. nov. Basionym; Clavulicium globosum Hjortstam & Ryvarden, Syn. Fung. (Oslo) 20∶35 (2005) MycoBank number:MB805765.
Stereopsidaceae Sjkvist, E. Larss., B.E. Pfeil & K. H. Larss., fam. nov. MycoBank number:MB805764.
Type Stereopsis D.A. Reid, Nova Hedwigia, Beih. 18: 290 (1965). Homobasidiomycetes with effused, stipitate, spathulate or funnel shaped sporocarps. Hymenium smooth. Hyphal system monomitic, with clamps. Basidia clavate, exemplar species with two sterigmata. Cystidia present. Spores hyaline, smooth, upon drying becoming angular. In soil or on living or dead wood. Exemplar species: Stereopsis radicans (Berk.) D.A. Reid and Stereopsis globosa (Hjortstam & Ryvarden) Sjökvist.
Stereopsidales Sjkvist, E. Larss., B.E. Pfeil & K. H. Larss., ord. nov. MycoBank number:MB805763.
Type Stereopsis D.A. Reid, Nova Hedwigia, Beih. 18: 290 (1965) Homobasidiomycetes with effused, stipitate, spathulate or funnel shaped sporocarps. Hymenium smooth. Basidia clavate, exemplar species with two sterigmata. Cystidia present. Spores hyaline, smooth, upon drying becoming angular. In soil or on living or dead wood. Exemplar species: Stereopsis radicans (Berk.) D.A. Reid and Stereopsis globosa (Hjortstam & Ryvarden) Sjkvist.
Morphology, Ecology, Life strategy and Distribution
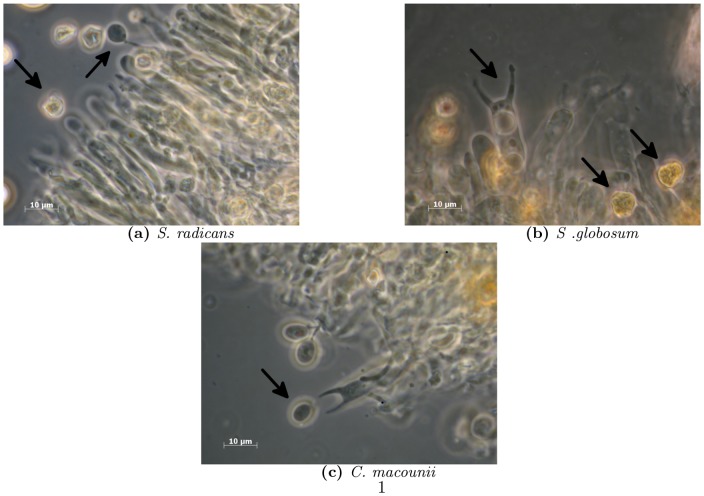
Morphological synapomorphies supporting the higher ranks of agaricomyceteous fungi are often absent and it appears as if many morphological traits are plastic or have evolved convergently in several lineages [3], [20], [21]. The corticioid fruiting body type, as seen in Clavulicium, is present in all orders of Agaricomycetes Dowell, but sometimes rare, like in the Phallomycetidae K. Hosaka, Castellano & Spatafora [6], [22]. Stipitate sporocarps with a smooth hymenophore that are characteristic of Stereopsis, are also present in Agaricales Underw., Thelephorales Corner ex Oberw. Hymenochaetales Oberw. and Polyporales Gum. Two-spored basidia, an apparent synapomorphy for the Stereopsis clade, are present in many other lineages of Agaricomycetes Dowell, e.g., in Atheliales Jlich and Agaricales Underw., but only in a few species. If Clavulicium macounii is sister species to the Stereopsis clade, a parsimony perspective would lead to the conclusion that the feature of two sterigmata has prevailed since the split between C. macounii and the Stereopsis clade. This clade would be up to 290 my, based on a comparison of a dated genome phylogeny [10] and the position of Hymenochaetales Oberw. and Auriculariales J. Schrt. The two sterigmata is an indication of an amphithallic reproductive mode [23], [24], where two nuclei are sorted to each spore, often omitting outcrossing. The highly refractive contents of the spores and the way in which the spores become angular and amber-like upon drying in Stereopsis radicans, S. globosa and C. macounii (fig 5a & b), is a morphological feature which separates them from species in other orders of Agaricomycetes Dowell.
Figure 5. Microscope images of hymenium structures.
Hymenium structures in 3% KOH showing basidiospores and palisades of basidia. a) Stereopsis radicans, arrows indicating developing spore (subglobose) attached to sterigmata, and mature dried (angular) spore. b) Stereopsis globosa, arrows indicating basidia with developed sterigmata, and dried angular spores. c) Clavulicium macounii arrow indicating free floating mature spore (cylindrical).
Stereopsis radicans and S. globosa are both found in tropical rain forest and cloud forest. According to [25] S. radicans is pantropically distributed, but we have only examined specimens from the neotropics. Stereopsis globosa has only been reported from Central and South America. The high molecular diversity observed among the limited number of specimens here referred to the morphological species S. radicans and S. globosa indicates that a high number of cryptic species may exist.
Clavulicium macounii is found on strongly decayed wood, mostly in boreal conifer forests, and like Stereopsis globosa it forms effused sporocarps with a smooth hymenophore. The micromorpological characters are the same as those in S. globosa and S. radicans, with the exception of the spore shape, which in C. macounii is ellipsoid (Fig 5).
Both Stereopsis and Clavulicium sensu lato display a considerable micromorphological diversity, for example in spore morphology, presence or absence of cystidia, and presence or absence of clamps. The specific spore morphology seen in Stereopsis radicans and S. globosa, are not known from other species of Stereopsis or Clavulicium. Whether any of those species currently classified as Stereopsis or Clavulicium that are not yet included in any molecular phylogenetic study could belong to Stereopsidales is hard to predict, there are no obvious candidates. Previous studies have found that S. vitellina belongs in Atheliales [7]; Stereopsis humphreyi belongs in Agaricales [7], [26]; a previous member of Clavulicium, Membranomyces delectabile, has been recovered in Cantharellales [3], [5], [21]. However, it is likely that further sampling in the tropics, focusing on corticioid and stipitate stereoid species will yield more species to add to Stereopsidales.
Materials and Methods
Taxon sampling
To place the species of study in an order or to verify the need for a new order for them, samples from all orders of Agaricomycetes [1], [2] were included in the dataset by three representatives, where available. GenBank sequences were from three recent molecular studies [2], [6], [27] listed in Table S1 in File S1. In addition, species which might be related to the study group were sought in public archives by using the BLAST algorithm [12], and the ten best matches for each gene were included in the datasets. Information on BLAST hits added to the dataset is listed in Table S2 in File S1.
PCR and sequencing
We amplified sequences from four nuclear genomic regions: tef1, rpb2, SSU and LSU. Thirty one sequences were newly generated for this study. For detailed information on the specimens see table 2. PCR amplifications were carried out using PuRe Taq Ready-to-go PCR beads (Amersham Biosciences, Uppsala) following the manufacturer€s recommendations. SSU was amplified using primer pairs NS1/NS4 and NS3/NS8 [28] 40 cycles using standard amplification parameters: initial denaturation in 95 C for 5 sec., and 94 C for 30 s, 60 C annealing temperature 30 s., and 72 C extension for 60 s. The amplification products were checked with electrophoresis for the presence of multiple products. Amplification products were purified using the QIA Quick PCR Purification Kit (Qiagen), following the manufacturers manual. The concentration of products was measured in a RNA/DNA calculator (Pharmacia biotech). Sequencing of the SSU region was performed was performed at Macrogen Incorporating (Korea), using primers pairs NS1, NS2, NS3, NS4, NS8 and NS51 [28]. Sequencing of tef1, rpb2 and LSU were performed as described in [29].
Table 2. Collection ID, Collection information and GenBank numbers of newly generated sequences.
| Taxon | Voucher (Herbarium/Collection) | Country | rpb2 | tef1 | nrDNA |
| Clavulicium globosum | GB/KHL12592 | Costa Rica | KC203501 | KC203515 | KC203495 |
| Clavulicium globosum | GB/KHL11228 | Costa Rica | KC203513 | KC203493 | |
| Clavulicium globosum | O/LR45201 | Belize | KC203509 | KC203489 | |
| Clavulicium sp | O/KHL12754 | Costa Rica | KC203510 | KC203490 | |
| Clavulicium sp. | O/KHL12949 | Costa Rica | KC203511 | KC203491 | |
| Clavulicium cf.globosum | O/KHL15523 | Brazil | KC203504 | KC203518 | KC203498 |
| Clavulicium macounii | GB/KHL12129 | Sweden | KC203514 | KC203494 | |
| Clavulicium macounii | GB/B.Nordn37145 | Sweden | KC203512 | KC203492 | |
| Clavulicium macounii | GB/KHL15566 | Sweden | KC203506 | KC203520 | KC203500 |
| Stereopsis cf. radicans | O/KHL15528 | Brazil | KC203503 | KC203517 | KC203497 |
| Stereopsis radicans | O/LR45395 | Belize | KC203502 | KC203516 | KC203496 |
| Stereopsis radicans | Cort/Baroni8943 | Venezuela | KC203507 | KC203487 | |
| Stereopsis radicans | GB/Ryvarden699 | Ecuador | KC203508 | KC203488 | |
| Stereopsis sp. | O/KHL15544 | Brazil | KC203505 | KC203519 | KC203499 |
Sequence editing, alignment and phylogenetic analyses
Sequences were assembled in Staden [30] using Pregap4 and Gap4, in Geneious (Geneious version 5.5.6 created by Biomatters available from http://www.geneious.com/) and in Sequencher 4.1 (Gene Codes Ann Arbor, Michigan) One sequence each of Stereopsis radicans and Clavulicium macounii were blasted using blastn [12] for each genetic marker (tef1, rpb2, SSU, and LSU, the combined SSU-LSU search only gave SSU hits), the top 10 blast hits for each were aligned to the respective dataset, and sequences that could not be aligned (one occurrence) were thereafter removed.
Sequences were aligned using Mafft [31] with default settings followed by manual inspection in Seaview [32]. Tef1and rpb2 sequences were blasted against RNA reference sequences in NCBI, aligned to two of the reference sequences, the introns removed, and the alignment trimmed at both ends. The tef1alignment was adjusted by eye in Seaview for four sequences. SSU was additionally aligned by structure in RNAsalsa [33] (using the structure model for Coprinus cinereus reference sequence M92991, found in the European ribosomal database at http://bioinformatics.psb.ugent.be/webtools/rRNA/). Ribosomal genes have complex secondary structures of loops and stems, where the nucleotide in a stem is paired with another nucleotide, whereas nucleotides in a loop are freely evolving. Thus any substitution in a stem will lead to another substitution, in the nucleotide pair. In a study on metazoan datasets [34] it was shown that in some cases structure aligned sequences in combination with a structure model perform better than analyses where secondary structure was not taken into account.
The datasets were analysed separately with Bayesian Markov chain Monte Carlo sampling and Maximum likelihood. Bayesian analyses were conducted in Mr Bayes 3.2.1 [35] using reversible model jump + 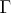 , with four parallel runs starting from random trees and sampling one tree every 1000 generations, and running until the standard deviation of split frequencies had stabilized under 0.05. Maximum likelihood was conducted in RaxML [36], [37] using GTR +
, with four parallel runs starting from random trees and sampling one tree every 1000 generations, and running until the standard deviation of split frequencies had stabilized under 0.05. Maximum likelihood was conducted in RaxML [36], [37] using GTR + 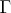 , and for the structure aligned SSU, RaxML was called with the consensus structure obtained from RNAsalsa, and Structure model 6A. The alignments and resulting trees are available at http://purl.org/phylo/treebase/phylows/study/TB2:S14833.
, and for the structure aligned SSU, RaxML was called with the consensus structure obtained from RNAsalsa, and Structure model 6A. The alignments and resulting trees are available at http://purl.org/phylo/treebase/phylows/study/TB2:S14833.
Recombination detection and SH-tests
Recombination tests were conducted in RDP4 [38] for each genetic marker separately. Settings used for RDP4 were as follows: an initial scan with RDP; using external and internal reference; window between 90–100%, Geneconv, Chimera, Maxchi, secondary bootscan and secondary siscan with default settings. Detected recombination events were rechecked with all methods, and the alignments for all recombination events were manually inspected.
We tested whether alternative groupings could be rejected by our data using SH topology tests [11]. These tests compare the log likelihood values of two competing hypotheses under a given p value. In this way it is possible to test whether one tree has a significantly higher likelihood than another. We wanted to know if the results of the individual gene trees for Stereopsis radicans, S. globosa and Clavulicium macounii, could be rejected in the conflicting trees, and whether the Stereopsis clade could be included in Polyporales.
SH tests were conducted in RaxML for each of the datasets to test several different hypotheses.
H1. Stereopsis radicans, Clavulicium macounii, S. globosa form a clade. H2. Phallomycetidae, S. radicans, C. macounii, and S. globosa form a clade. H3. Phallomycetidae is monophyletic; S. radicans, C. macounii, S. globosa are monophyletic, thus Phallomycetidae and Stereopsis - Clavulicium are reciprocally monophyletic. H4. Phallomycetidae and C. macounii form a clade. H5. Polyporales and S. radicans, S. globosa and C. macounii form a clade. The best tree for each genetic marker was used as H0, whereas H1, H2, H3, H4 and H5 were tested in turn unless redundant.
Specimens examined
Stereopsis radicans, Surinam, 1879, K(M) 178844 (KEW), TYPE. Stereopsis radicans, Venezuela, Aragua st, 30 August 1999, Soil, Baroni8943 (CORT). Stereopsis radicans, Ecuador, Napo, Santa Rosa de Quijos, 12 Feb. 1980, On fallen trunk, Montane rain forest alt. ca 1500 m, Ryvarden699 (GB). Stereopsis radicans, Belize, Cayo district, Five sisters, Nature trail, 2 November 2002, On dead decidious wood, LR45395(O). Stereopsis sp. Brazil, On the ground, KHL15544 (O). Stereopsis cf. radicans, Brazil, On living tree, KHL 15528 (O). Stereopsis cf. globosum, Brazil, On living tree, KHL15523 (O). Clavulicium globosum, Belize, Cayo district, Blue Hole Nat. Park, Hummingbird trail, 28 October 2002, On dead decidious wood, LR45201 (O). Clavulicium sp. Costa Rica, Puntarenas, Coto Brus, Sabalito, Zona Protectora Las Tablas La Neblina, 5 Nov 2004, On angiosperm log, Alt. ca 1350 m, KHL12754 (O). Clavulicium sp. Costa Rica, San Jose, Dota, San Gerardo, Sendro la Quebrada, 9 Nov 2004, On strongly decayed wood of angisperm tree, Alt. ca 2400, KHL12949 (O). Clavulicium globosum, Costa Rica, Wood, KHL 11228. Clavulicium sp. Costa Rica, Puntarenas Coto Brus, Sabalito, Zona Protectora Las Tablas, Progreso, Camino a Cotoncito, 3 November 2004, On wood, Alt. 1560, KHL12592 (GB). Clavulicium globosum, Ecuador, Orellana prov, Yasuni National Park, Yasuni Scientific research station, On dead wood, March 2002, Ryvarden 44705, HOLOTYPE. Clavulicium macounii, Norway, Nordland, Hemnes, Sjforsen nat. res. Neverbekken, On Picea abies, 25 August 2012, KHL 15620 (O). Clavulicium macounii, Sweden, Vstergaland, dens par., S of the church, not far from ren, 18 Oct, 2003, Picea abies trunk, KHL 12129 (GB). Clavulicium macounii, Quebec, Hull, J, Macoun, No 368 Oct, 17 1898 Ex Herb, E, A, Burt, TYPE.
Nomenclature
The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants, and hence the new names contained in the electronic publication of a PLOS ONE article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies.
In addition, new names contained in this work have been submitted to MycoBank from where they will be made available to the Global Names Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix http://www.mycobank.org/MB/. The online version of this work is archived and available from the following digital repositories:PubMed Central, LOCKSS, GUPEA.
Supporting Information
Tables S1–S2. Table S1, Genbank numbers of public sequences used in this study. Table S2, Significant BLAST hits.
(PDF)
Acknowledgments
We are grateful to Emelie Lindquist for help in the lab.
Funding Statement
KHL and EL have financial support from Svenska artprojektet, Artdatabanken, SLU. ES is supported by grants from Kapten Carl Stenholms fond, Wilhelm och Martina Lundgrens fond and Anna och Gunnar Vid-felts fond för biologisk forskning. BEP is supported by grants from the Swedish Research Council (2009–5206), the Royal Swedish Academy of Sciences, Lars Hiertas Minne fund, The Royal Physiographic Society in Lund, Helge Ax:son Johnsons fund and the Lundgrenska fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, et al. (2007) A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509–47. [DOI] [PubMed] [Google Scholar]
- 2. Binder M, Larsson KH, Matheny PB, Hibbett DS (2010) Amylocorticiales ord. nov. and Jaapiales ord. nov.: Early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102: 865–880. [DOI] [PubMed] [Google Scholar]
- 3. Larsson KH, Larsson E, Kõljalg U (2004) High phylogenetic diversity among corticioid homoba284 sidiomycetes. Mycological Research 108: 983–1002. [DOI] [PubMed] [Google Scholar]
- 4. Matheny PB, Curtis JM, Denitis M, Way H, Carolina N, et al. (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98: 982–995. [DOI] [PubMed] [Google Scholar]
- 5. Moncalvo JM, Nilsson RH, Koster B, Dunham SM, Bernauer T, et al. (2006) The cantharelloid clade: dealing with incongruent gene trees and phylogenetic reconstruction methods. Mycologia 98: 937–948. [DOI] [PubMed] [Google Scholar]
- 6. Hosaka K, Bates ST, Beever RE, Castellano MA, Colgan W, et al. (2006) Molecular phylogenetics of the gomphoid-phalloid fungi with an establishment of the new subclass Phallomycetidae and two new orders. Mycologia 98: 949–59. [DOI] [PubMed] [Google Scholar]
- 7. Sjökvist E, Larsson E, Eberhardt U, Ryvarden L, Larsson KH (2012) Stipitate stereoid basidiocarps have evolved multiple times. Mycologia 104: 1046–55. [DOI] [PubMed] [Google Scholar]
- 8. Maureira-Butler IJ, Pfeil BE, Muangprom A, Osborn TC, Doyle JJ (2008) The reticulate history of Medicago (Fabaceae). Systematic Biology 57: 466–82. [DOI] [PubMed] [Google Scholar]
- 9. Philippe H, Brinkmann H, Lavrov DV, Littlewood DTJ, Manuel M, et al. (2011) Resolving difficult phylogenetic questions: why more sequences are not enough. PLoS Biology 9: e1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Floudas D, Binder M, Riley R, Barry K, Blanchette RA, et al. (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science (New York, NY) 336: 1715–9. [DOI] [PubMed] [Google Scholar]
- 11. Shimodaira H, Hasegawa M (1989) Letter to the Editor Multiple Comparisons of Log-Likelihoo302 ds with Applications to Phylogenetic Inference. Molecular Biology and Evolution 16: 1114–1116. [Google Scholar]
- 12. Altschul SF, Warren G, Webb M, Myers WE, Lipman DJ (1990) Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 13. Padidam M, Sawyer S, Fauquet CM (1999) Possible emergence of new geminiviruses by frequent recombination. Virology 265: 218–25. [DOI] [PubMed] [Google Scholar]
- 14.Posada D, Crandall KA (2001) Evaluation of methods for detecting recombination from DNA sequences: Computer simulations. Proceedings of the National Academy of Sciences of the United States of America 98.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith JM (1992) Analyzing the Mosaic Structure of Genes. Journal of Molecular Evolution 34: 126–129. [DOI] [PubMed] [Google Scholar]
- 16. Martin DP, Posada D, Crandall KA, Williamson C (2005) A Modified Bootscan Algorithm for Automated Identification of Recombinant Sequences and Recombination Breakpoints. Aids Research and Human Retroviruses 21: 98–102. [DOI] [PubMed] [Google Scholar]
- 17. Gibbs MJ, Armstrong JS, Gibbs AJ (2000) Sister-Scanning: a Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 16: 573–582. [DOI] [PubMed] [Google Scholar]
- 18.Kirk PM, Cannon PF, Minter DW, Stalpers JA, editors (2008) Dictionary of the Fungi. 10 edition.
- 19. Ryberg M, Kristiansson E, Sjökvist E, Nilsson RH (2009) An outlook on the fungal internal transcribed spacer sequences in GenBank and the introduction of a web-based tool for the exploration of fungal diversity. The New Phytologist 181: 471–7. [DOI] [PubMed] [Google Scholar]
- 20. Hibbett DS, Binder M (2002) Evolution of complex fruiting-body morphologies in homobasidiomycetes. Proceedings Biological sciences/The Royal Society 269: 1963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Binder M, Hibbett DS, Larsson K, Larsson E, Langer E, et al. (2005) The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (Homobasidiomycetes). Systematics and Biodiversity 3: 113–157. [Google Scholar]
- 22. Larsson KH (2007) Re-thinking the classification of corticioid fungi. Mycological Research 111: 1040–63. [DOI] [PubMed] [Google Scholar]
- 23. Petersen RH (1976) Mycological Society of America Studies on Nuclear Division and Behavior within Basidia I. Hydnum umbilicatum. Mycologia 68: 666–672. [Google Scholar]
- 24. Petersen RH (1995) There's More to a Mushroom than Meets the Eye: Mating Studies in the Agaricales. Mycologia 87: 1–17. [Google Scholar]
- 25.Reid DA (1965) A monograph of the Stipitate stereoid fungi. Nova Hedwigia. Weinheim: Cramer. Key: Reid1965 Annotation: Monograph
- 26. Moncalvo JM, Vilgalys R, Redhead Sa, Johnson JE, James TY, et al. (2002) One hundred and seventeen clades of euagarics. Molecular Phylogenetics and Evolution 23: 357–400. [DOI] [PubMed] [Google Scholar]
- 27. Matheny PB, Wang Z, Binder M, Curtis JM, Lim YW, et al. (2007) Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Molecular Phylogenetics and Evolution 43: 430–51. [DOI] [PubMed] [Google Scholar]
- 28.White T, Bruns T, Lee S, Taylor S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic Press, New York, 315–322 pp.
- 29. Miettinen O, Larsson E, Sjökvist E, Larsson K (2012) Comprehensive taxon sampling reveals unaccounted diversity and morphological plasticity in a group of dimitic polypores. Cladistics 28: 251–270. [DOI] [PubMed] [Google Scholar]
- 30. Staden R (1996) The Staden sequence analysis package. Molecular biotechnology 5: 233–41. [DOI] [PubMed] [Google Scholar]
- 31. Katoh K, Misawa K, Kuma Ki, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galtier N, Gouy M, Gautier C (1996) SEA VIEW and PHYLO_ WIN: two graphic tools for sequence alignment and molecular phylogeny. Bioinformatics (Oxford, England) 12: 543–548. [DOI] [PubMed] [Google Scholar]
- 33.Stocsits RR, Letsch H, Hertel J, Misof B, Stadler PF (2009) Accurate and efficient reconstruction of deep phylogenies from structured RNAs – Supplemental Material –. Technical Report June. [DOI] [PMC free article] [PubMed]
- 34. Letsch HO, Kjer KM (2011) Potential pitfalls of modelling ribosomal RNA data in phylogenetic tree reconstruction: evidence from case studies in the Metazoa. BMC Evolutionary Biology 11: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, et al. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics (Oxford, England) 22: 2688–90. [DOI] [PubMed] [Google Scholar]
- 37. Ott M, Zola J, Stamatakis A, Aluru S (2007) Large-scale maximum likelihood-based phylogenetic analysis on the IBM BlueGene/L. Proceedings of the 2007 ACM/IEEE conference on Supercomputing - SC' 07: 1. [Google Scholar]
- 38. Martin DP, Lemey P, Lott M, Moulton V, Posada D, et al. (2010) RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics (Oxford, England) 26: 2462–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2. Table S1, Genbank numbers of public sequences used in this study. Table S2, Significant BLAST hits.
(PDF)