Figure 1.
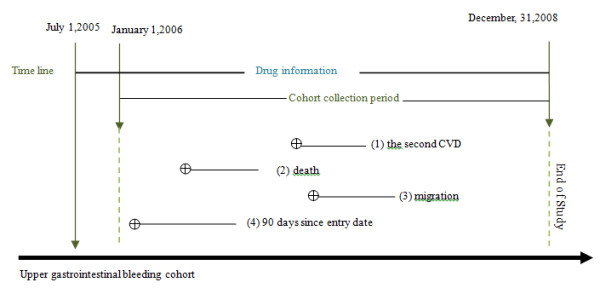
The flow chart for study design. The timeline showed how the cohort was defined. The Swedish Prescribed Drug Register started on July 1st, 2005. This study chose to start on January, 1st 2006 thus at least half-year drug exposure information can be guaranteed. Patients who discharged from hospitalizations for cardiovascular diseases since January, 1st 2006 were included in the cohort. They had been followed up the second cardiovascular event (1), death (2), migration (3), or end of study (4) within 90 days follow-up period. All of the patients had historical upper GI bleeding or they bled during/after the follow up period but before December, 31, 2008. Symbol ⊕ means diagnosis of cardiovascular diseases.
