Abstract
We have recently identified two genes coding for ammonium transporters (AMT) in Sorghum bicolor that were induced in roots colonized by arbuscular mycorrhizal (AM) fungi. To improve our understanding of the dynamics of ammonium transport in this symbiosis, we studied the transfer of soil-ammonium-derived 15N to S. bicolor plants via the Glomus mosseae fungal mycelium in compartmented microcosms. The 15NH4+-containing hyphal compartment was inaccessible to the roots in the plant compartment. 15N label concentrations significantly increased in plant roots and leaves already 48 h after exposure of the AM fungus to the 15NH4+ substrate, attesting an efficient symbiotic N transfer between the symbiotic partners and further highlighting that AM symbiosis represents an important component of plant nitrogen nutrition.
Keywords: nitrogen, mycelium, arbuscular mycorrhizal fungi, transfer, sorghum, 15N labeling
We are interested in the arbuscular mycorrhizal (AM) symbiosis1,2 using Glomus mossea-inoculated sorghum (Sorghum bicolor) as a model system. Recently, we described the expanded family of ammonium transporters (AMTs) in sorghum.3 We could demonstrate that two AMTs, SbAMT3;1 and SbAMT4, are upregulated in mycorrhized plants (AM-inducible AMTs) and that SbAMT3;1 is localized at the plant-fungus interface. However, the biological significance of ammonium transport during symbiotic interaction remained elusive.
Nitrate and ammonium are two main sources of bioavailable inorganic N in soils. Albeit the high mobility of nitrate and ammonium in soils, fixed N-depletion (in the vicinity of the root system) is a common environmental constraint plants have to cope with.2 Different studies estimated that AM fungi delivered between 30% and 42% of the N taken up by the plant.4,5 Mitigation of N deficiency through the interaction with AM fungi may play a particularly important role under drought-stress conditions or in marginal soils were nutrient supply is strongly limited,1 environmental conditions that are often pertinent to the growth of sorghum. In fact, sorghum can grow under more arid conditions than most other grain crops, making it an existential source of food, feed or fiber for many farmers living in the semi-arid tropics of Africa, Asia and South America.6
To determine the importance of AM fungi in N nutrition of sorghum, we set up an experiment using compartmented microcosms (Fig. 1), where one plant and one hyphal compartment are connected, but separated by two 21 μm nylon meshs and an air gap in between. The air gap was created by placing two 5 mm plastic meshs between the two 21 μm nylon meshs. The two compartments were filled with sterile (120°C, 20 min) growth substrate consisting of a mixture of Terragreen (American aluminum oxide, oil-dry US special, type III R, 0.125 mm; Lobbe Umwelttechnik), sand (quartz sand from Alsace, 0.125–0.25 mm; Kaltenhouse) and Loess from a local site (5:4:1, w/w/w). Sorghum [S. bicolor (L.) Moench], cv Pant-5 seedlings were inoculated with a 2 g (approximately 100 spores) inoculum of G. mosseae ISCB13 or with 2 g of sterilized (120°C, 20 min) inocula as a non-mycorrhizal control. In the center of the hyphal compartment, a 21 μm nylon mesh bag of 15 ml was inserted and kept empty until introduction of the 15N labeled substrate 12 wk after inoculation. Then the nylon mesh bag was filled with 13 g of sand including 10 mg of 15N ammonium sulfate (Cambridge Isotope Laboratory). Two ml of water were added to wet the 15N-spiked sand without inducing mass flow. The microcosms were irrigated with distilled water twice a week. In addition, the compartments were amended weekly with 8 mL of a Hoagland solution.3 Plants were grown under controlled conditions (16 h of light at 28°C and 8 h of dark at 15°C, constant relative aerial humidity of 65%).
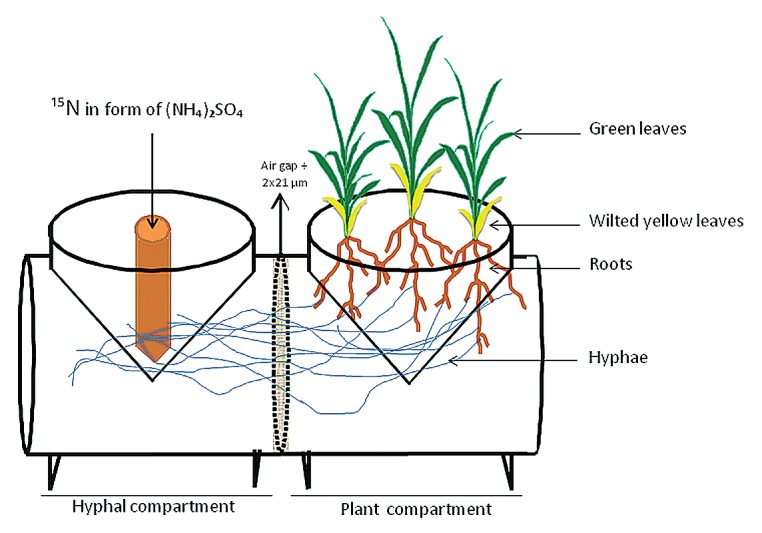
Figure 1. Compartmented microcosms. One plant and one hyphal compartment are separated by two 21 μm nylon meshes and an air gap to prevent mass flow. A 21 μm nylon mesh bag of 15 ml was inserted in the center of the hyphal compartment and filled with 13 g of sand including 10 mg of 15N ammonium sulfate 12 wk after inoculation.
A total of 40 microcosms were prepared. Four compartmented microcosms were harvested separately 0, 12, 24, 48 and 72 h after 15N-labeling. From the plant compartments, roots, green parts of the leaves and wilted, yellow leaves were harvested separately. In the mycorrhized systems, the AM fungal mycelium was harvested by picking both in the plant and in the hyphal compartment. The total amount of N and 15N/14N ratio of plant materials were determined using an elemental analyzer (EA) and a ThermoFinnigan DeltaV Advantage Continuous-Flow EA-IRMS, respectively. For N isotope analyses, up to 2 mg of bulk sample was combusted at 1,030°C, combustion gases were passed through a reduction column (650°C) and produced N2 gas was purified and transferred to the IRMS. Isotope values were calibrated using internal EDTA (−1.1 per mil) and ammonium oxalate (+32.7 per mil) standards, both of which had previously been calibrated against international standards.
Transfer of excess (i.e., beyond natural abundance) 15N to the non-mycorrhized plants was undetectable (Table S1). On the other hand, 15N-label was found already 24 h after spiking in the AM fungal mycelium from both the hyphal and the plant compartment, indicating rapid ammonium uptake by AM fungi (Fig. 2; Table S1). Transfer of mycorrhizal N to the host plant is indicated by the increase of 15N in the plants. In fact, the 15N turnover from the substrate pool to the plant was significantly higher in the mycorrhized compared with the non-mycorrhized plant systems (p = 0.00, f = 40.83) after 48 h (Table S1). Label 15N was present both in roots and in green leaves, indicating a substantial transfer to the entire plant, except to the wilted yellow leaves, which did not display any marked increase in 15N/14N ratio (Figs. 2 and 3; Table S1). The 15N concentrations continued to increase between 48 and 72 h, indicating ongoing transfer during the experimental period (Fig. 2; Table S1) and 15N was accumulating in the plant (Fig. 3).
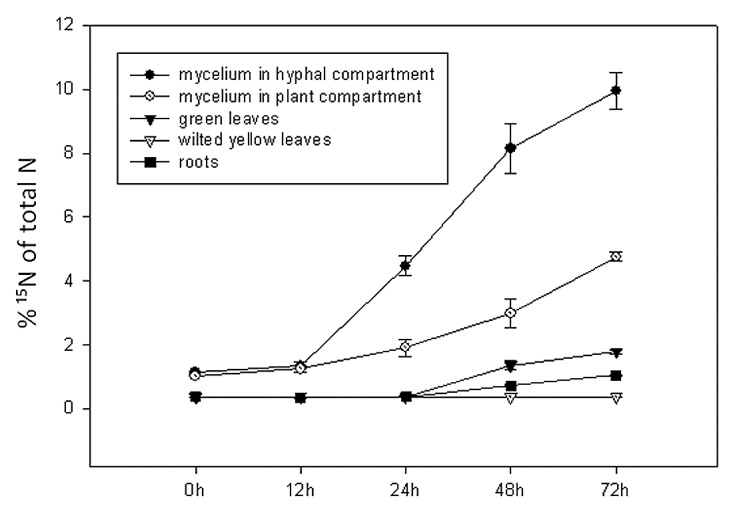
Figure 2. Time course experiment on the transfer of (15NH4)2SO4 as N source from the soil to Sorghum bicolor plants via Glomus mosseae ISCB13 mycelium.15N was measured in different tissues after 0, 12, 24, 48 and 72 h of labeling: roots, green leaves and wilted, yellow leaves of plants as well as in the AM fungal mycelium from the plant and from the hyphal compartment. Values are the means of four replicates.
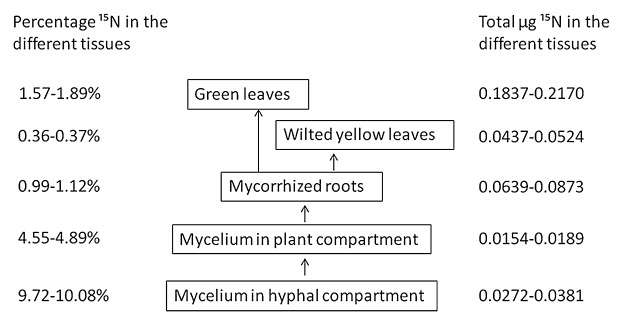
Figure 3. Percentage of 15N and total 15N (μg) in the different tissues of mycorrhized sorghum plants harvested in the compartmented microcosms after 72 h of labeling.Values represent the mean of four replicate treatments.
AM fungi are able to take up N is form of nitrate, ammonium or amino acids from the soil far away from the plant5,7 and they are believed to transfer it to the arbuscules, through the extraradical mycelium, in the form of arginine.8 There, they may produce ammonium and deliver it to the plant, which is then taken up by plants’ AMTs induced upon colonization by AM-inducible AMTs at the symbiotic interface. Our data underline the effectiveness of AM fungi in the uptake of soil ammonium and symbiotic N transfer. It still remains to be seen what role the AM-inducible AMTs play during symbiotic N incorporation.
Supplementary Material
Acknowledgments
This project was supported by the Swiss National Science Foundation (grant nos. 130794 to A.W. and PZ00P3_136651 to P-E.C. and the R’Equip grant no. 121258 to M.F.L. and T.B.). We thank Dr Mark Rollog for laboratory assistance and scientific support in the stable isotope lab.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25229
References
- 1.Smith SE, Read DJ. Mycorrhizal Symbiosis. Cambridge, UK: Academic Press, 2008. [Google Scholar]
- 2.Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol. 2011;62:227–50. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- 3.Koegel S, Ait Lahmidi N, Arnould C, Chatagnier O, Walder F, Ineichen K, et al. The family of ammonium transporters (AMT) in Sorghum bicolor: two AMT members are induced locally, but not systemically in roots colonized by arbuscular mycorrhizal fungi. New Phytol. 2013;198:853–65. doi: 10.1111/nph.12199. [DOI] [PubMed] [Google Scholar]
- 4.Mader P, Vierheilig H, Streitwolf-Engel R, Boller T, Frey B, Christie P, et al. Transport of N-15 from a soil compartment separated by a polytetrafluoroethylene membrane to plant roots via the hyphae of arbuscular mycorrhizal fungi. New Phytol. 2000;146:155–61. doi: 10.1046/j.1469-8137.2000.00615.x. [DOI] [Google Scholar]
- 5.Govindarajulu M, Pfeffer PE, Jin HR, Abubaker J, Douds DD, Allen JW, et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature. 2005;435:819–23. doi: 10.1038/nature03610. [DOI] [PubMed] [Google Scholar]
- 6.Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–6. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 7.Leigh J, Hodge A, Fitter AH. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009;181:199–207. doi: 10.1111/j.1469-8137.2008.02630.x. [DOI] [PubMed] [Google Scholar]
- 8.Tian CJ, Kasiborski B, Koul R, Lammers PJ, Bücking H, Shachar-Hill Y. Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: gene characterization and the coordination of expression with nitrogen flux. Plant Physiol. 2010;153:1175–87. doi: 10.1104/pp.110.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


