Abstract
In the present study, we investigated the effects of dopamine, an allelochemical exuded from the velvetbean (Mucuna pruriens L DC. var utilis), on the growth and cell viability of soybean (Glycine max L. Merrill) roots. We analyzed the effects of dopamine on superoxide dismutase, phenylalanine ammonia-lyase and cell wall-bound peroxidase activities as well as its effects on lignin contents in the roots. Three-day-old seedlings were cultivated in half-strength Hoagland nutrient solution (pH 6.0), without or with 0.25 to 1.0 mM dopamine, in a growth chamber (25°C, 12L:12D photoperiod, irradiance of 280 μmol m−2 s−1) for 24 h. In general, the length, fresh weight and dry weight of roots, cell viability, PAL and POD activities decreased, while SOD activities increased after dopamine treatment. The content of lignin was not altered. The data demonstrate the susceptibility of soybean to dopamine and reinforce the role of this catecholamine as a strong allelochemical. The results also suggest that dopamine-induced inhibition in soybean roots is not related to the production of lignin, but may be related to damage caused by reactive oxygen species.
Keywords: allelopathy, dopamine, lignin, peroxidase, phenolic compounds, phenylalanine ammonia-lyase, superoxide dismutase, root growth, soybean
Introduction
Plants can release organic compounds into the environment. These secondary metabolites may accumulate in the soil environment and influence the growth and development of neighboring plants, with positive and negative effects. This is called allelopathy, a natural ability of plants to protect themselves through natural allelochemicals.1 Allelochemicals typically inhibit seed germination and seedling growth. Moreover, they alter several physiological and biochemical processes including water utilization, mineral uptake, foliar expansion, photosynthesis, amino acid metabolism, protein synthesis, glycolysis, mitochondrial respiration and ATP synthesis, among others.2 Dopamine is one of these compounds; it is widespread in animals and has also been detected in many plant families.3
Velvetbean [Mucuna pruriens (L.) DC. var utilis] is widely used in tropical regions for intercropping with maize, sorghum and millet and for providing benefits, such as suppression of the nematode population, weed smothering, symbiotic nitrogen fixation, nutrient recycling and control of erosion.4 Many secondary compounds are produced by velvetbean. Using HPLC coupled with mass spectrometry, Wichers et al.5 identified dopamine in 2–3-week-old leaves of Mucuna. The dopamine content of the leaves even exceeded the content of L-DOPA, the most abundant allelochemical in Mucuna. However, in the roots, stems and seeds, no dopamine could be detected at any stage of development. Matsumoto6 reported that mucuna metabolizes L-DOPA to dopamine in leaves as a protective mechanism against the toxicity of L-DOPA. Dopamine has also been detected in many other plant families. Yue et al.7 demonstrated that noradrenaline and dopamine are major bioactive components of Portulaca oleracea L., a traditional herbal medicine. Dopamine is found at high concentrations in potato (Solanum tuberosum) plants, the spathes of Araceae inflorescences, as well as the pulp of yellow banana (Musa acuminata), red banana (Musa sapientum), plantain (Plantago major) and fuerte avocado (Persea americana).3,8
The role of dopamine in plants is poorly documented. It has been proposed to be a precursor for various alkaloids benzylisoquinolines like papaverine and morphine or of the hallucinogenic alkaloid mescaline.9 Some studies have addressed the effects of dopamine on plants and have revealed that it has attributes typical of an allelochemical. Dopamine is associated with defense against herbivores, processes such as nitrogen fixation, flowering and prevention of IAA oxidation, intercellular regulation of ion permeability and photophosphorylation of chloroplasts.10-13 Exogenous dopamine at concentrations of 5–100 μM stimulates ethylene biosynthesis in illuminated chloroplast lamellae from sugar beet leaves.14
Dopamine is formed in plants from tyrosine, either via tyramine or via L-DOPA.3 The physiological mechanism of action of dopamine in plants, however, is poorly understood. In animals, dopamine is a well-known neurotransmitter; its absence in nerve cells can cause Parkinson disease. One of the more important chemical changes is dopamine auto-oxidation or oxidation by enzymes, leading to melanins. During oxidation, both semiquinones and quinones are generated in a chain autoxidation process, which also results in the production of ROS like 1O2, O2−, HO● and H2O2.6,15 These ROS, as well as semiquinone and quinone products of catecholamine oxidation, can interact with proteins, lipids, nucleic acids and membrane components, thus causing cell damage.
The phenylpropanoid pathway is one of the most important metabolic pathways since it synthesizes phenolic compounds and a wide range of secondary products in plants, including lignin. PAL is regarded as the primary enzyme of the phenylpropanoid biosynthetic pathway, wheareas POD within the cell wall, in either the free or bound state, has been shown to be associated with monolignol polymerization and, therefore, is important in lignin synthesis.16 To date, no reports on the effects and mode of action of exogenous dopamine on soybean roots are available. Due to the important role of lignification in root growth, the question addressed in the current research was whether dopamine affects PAL and cell wall-bound POD activities, as well as the lignin content of soybean roots.
Results
Root fresh and dry weights and root lengths decreased with increasing concentrations of dopamine in soybean seedlings grown during exposure (24 h) (Table 1). Mean total root lengths were 9.2, 29.5 and 48% less than the controls for the 0.25, 0.5 and 1.0 mM dopamine. Root fresh weight decreased by 8.8, 20, and 31.1% at the 0.25, 0.5 and 1.0 mM treatments, respectively, compared with the control. Similar behavior was observed in root dry weights, which were found to be 13.4, 19.8 and 37.6% less than those of the controls with 0.25, 0.5 and 1.0 mM dopamine.
Table 1. Changes in root length root fresh and dry weights of soybean seedlings treated with dopamine for 24 h.
| Dopamine(mM) | Root length (cm) | % | Fresh weight (g) | % | Dry weight (g) | % |
|---|---|---|---|---|---|---|
| 0 | 2.338 ± 0.167 | 2.393 ± 0.080 | 0.141 ± 0.005 | |||
| 0.25 | 2.123 ± 0.384ns | 9.19 | 2.181 ± 0.118* | 8.86 | 0.122 ± 0.004* | 13.48 |
| 0.50 | 1.648 ± 0.105* | 29.51 | 1.913 ± 0.023* | 20.06 | 0.113 ± 0.002* | 19.86 |
| 1.00 | 1.216 ± 0.028* | 47.99 | 1.729 ± 0.080* | 31.09 | 0.088 ± 0.008* | 37.59 |
Means (n = 4 ± SE) significantly smaller than the experiment control (Dunnett’s multiple comparison test) are marked *. ns = not significant at 0.05 level. The symbol % represents inhibition of statistically significant means when compared with control (0 mM).
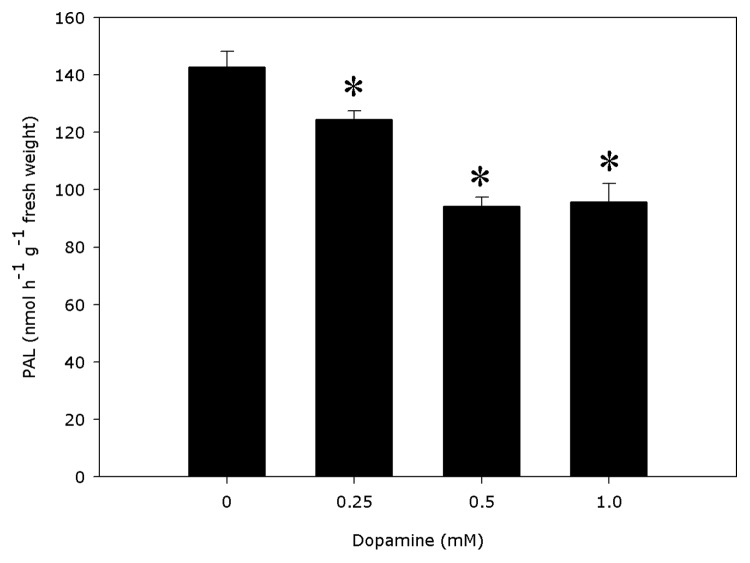
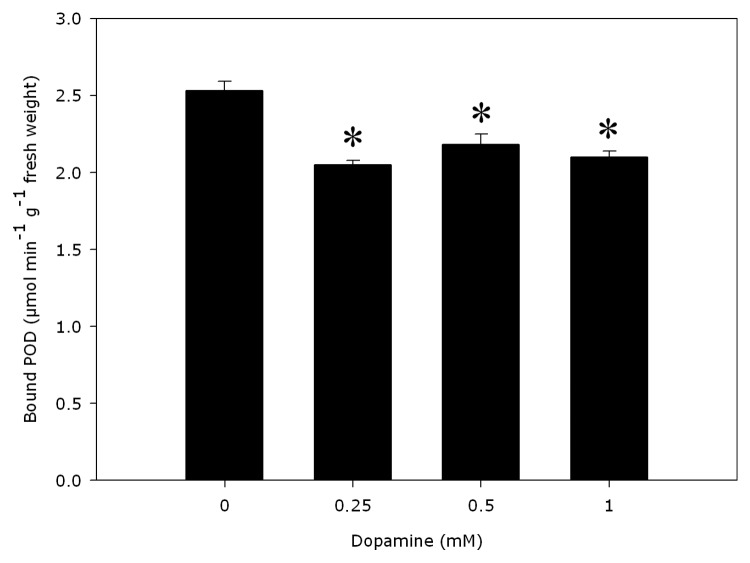
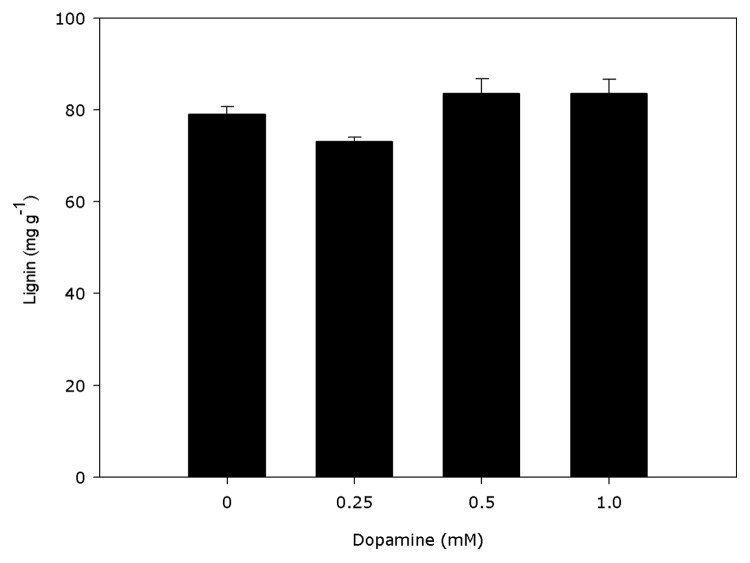
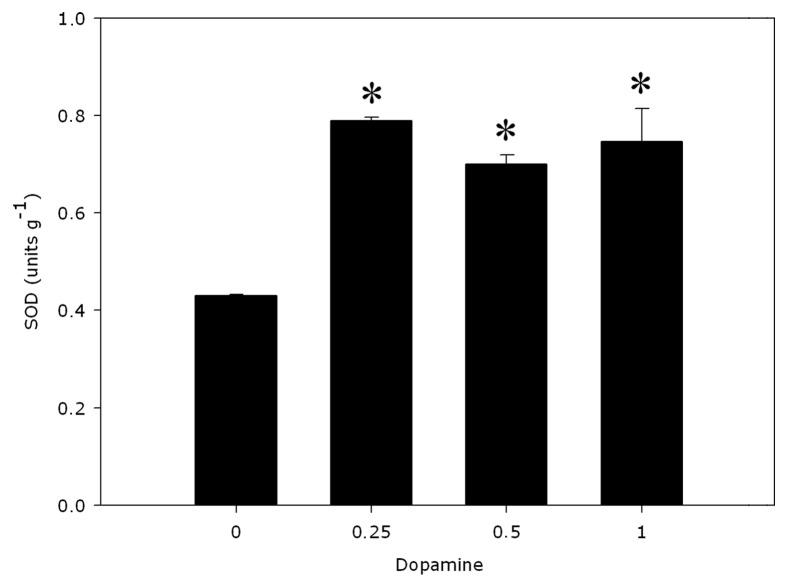
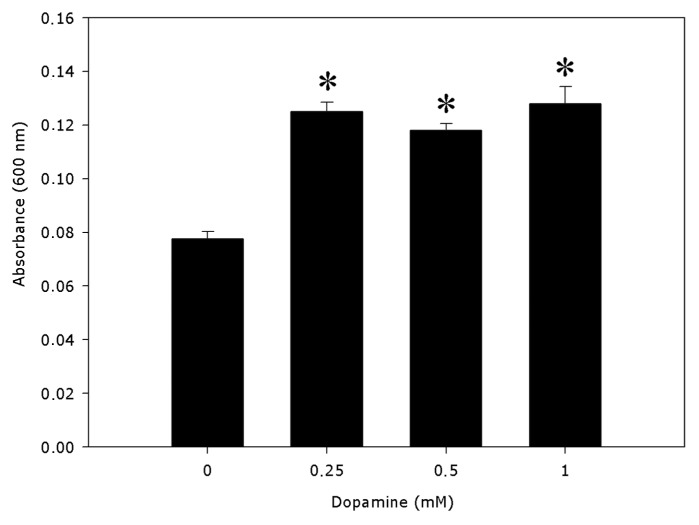
PAL activities were significantly distinct from the controls (Fig. 1). Dopamine decreased the activity of PAL by 12.8% to 34% with 0.25 to 1.0 mM dopamine. Cell wall-bound POD activities (Fig. 2) decreased 16.3% indifferent of the dopamine concentration and no significant modify was detected in the lignin content (Fig. 3), compared with control. The data reveal that 0.25 to 1.0 mM dopamine activated SOD by 73.2% on average, in relation with the control (Fig. 4). Finally, it was found that the root cell was also affected significantly by exposure to dopamine. A loss of cell viability was increased by around 59.7% compared with the control (Fig. 5).
Figure 1. Effects of dopamine on phenylalanine ammonia-lyase (PAL). *Values (n = 3 ± SE) differ statistically (Dunnett’s multiple comparison test) from control (p < 0.05).
Figure 2. Effects of dopamine cell wall-bound peroxidases (POD). *Values (n = 4 ± SE) differ statistically (Dunnett’s multiple comparison test) from control (p < 0.05).
Figure 3. Effects of dopamine on lignin contents. *Values (n = 5 ± SE) differ statistically (Dunnett’s multiple comparison test) from control (p < 0.05).
Figure 4. Effects of dopamine on SOD. *Values (n = 3 ± SE) differ statistically (Dunnett’s multiple comparison test) from control (p < 0.05).
Figure 5. Loss of cell viability in the roots of the soybean seedlings treated with dopamine. *Values (n = 3 ± SE) differ statistically (Dunnett’s multiple comparison test) from control (p < 0.05).
Discussion
The main outcome exposed in the present study is that root growth (Table 1), PAL (Fig. 1), cell wall-bound peroxidase activity (Fig. 2) and cell viability (Fig. 5) decreased after dopamine treatment, while SOD activity increased (Fig. 4) and the lignin content (Fig. 3) was not changed.
The discovery that dopamine did not stimulate lignin production (Fig. 3) is in agreement with the observed inhibition of PAL (Fig. 1) and cell wall-bound peroxidase activities (Fig. 2). The reduction in seedling roots has been related with lignification of the cell walls and increases in PAL and POD activities and phenolic compounds.17-20 PAL is regarded as the access enzyme into the phenylpropanoid pathway and catalyzes the synthesis of phenolic compounds. In relation to cell wall-bound POD, there is long-standing evidence that this enzymatic form causes oxidative polymerization of monolignols from phenolic compounds, causing cell wall lignification and resulting in growth reduction.16 Although root growth of soybean was reduced by dopamine (Table 1), the enzymatic activities of PAL and cell wall-bound POD (Figs. 1 and 2) also declined, with no increase in the production of lignin (Fig. 3). Thus, our results suggest that dopamine-induced inhibition in soybean roots does not appear to be related to the synthesis of lignin.
Changes in the growth of roots by dopamine have been reported in a few plant species. Protacio et al.21 showed that catecholamines caused a stimulation of growth in root cultures of Acmella oppositifolia and Nicotiana tabacum cultures. According to the authors, dopamine affects plant development by acting with hormones, leading in elevated contents of auxin. It was shown that dopamine can inhibit IAA oxidation in vitro as well as in vivo via the inhibition of IAA oxidase.12 It is known that auxins promote the growth of stems and coleoptile and inhibit the growth of roots. It is likely that roots may require a minimum concentration of auxin to grow, but growth is strongly inhibited by concentrations of auxin required to promote elongation of stems and coleoptile.22 Thus, if dopamine actually inhibits IAA oxidase, thereby increasing the auxin content and high levels of this hormone in the roots inhibit growth, it is no exaggeration to suggest that this could be one of the modes of action of dopamine applied to the roots of soybean seedlings.
Other studies have indicated that the dopamine toxicity may be related to its oxidation. This compound can be enzymatically or spontaneously metabolized by molecular oxygen in physiological solutions to form ROS, leading to the formation of melanins. Melanin biosynthesis is also linked to other dopamine oxidation substances, like quinones and semiquinones.6,17,23,24 Low concentrations of these ROS can stimulate several cellular processes and regulate several important physiological functions. Moreover, ROS, semiquinone and quinones can interact with proteins by denaturing them, thus causing injury to DNA and the cell membrane.15,25,26 To confirm whether melanin was generated from dopamine, soybean seedlings were developed with this compound (0.25–1.0 mM) in a nutrient solution. Our data (not shown) reveal that roots in contact to dopamine synthesized a significant quantity of melanin.
As a method to indirectly assess possible increased levels of ROS like O2−, we determined the activity of SOD (Fig. 4). SOD enzymes are famous scavengers of O2−. Takano et al.27 observed the effects of dopamine on extracellular SOD expression in cultured rat cortical astrocytes. SOD was increased by 24 h of dopamine exposure in a dose-dependent manner. Dopamine has been shown to markedly reduce the neuroblastoma cells in the cerebrum of Parkinson disease patients and the production of dopamine semiquinone was observed in the brains of Parkinson disease patients by electron spin resonance spectrometry but semiquinone generation and toxicity were avoided by SOD, indicating the participation of superoxide radicals as the mechanism of toxicity.6 In our studies, SOD activities were significantly increased (Fig. 4), suggesting a possible increase in the levels of O2− in the roots of seedlings exposed to dopamine.
Several studies have found that the cytotoxicity of dopamine, in animal cells, is dependent on ROS. Lai and Yu28 demonstrated a correlation between ROS that broke plasma membrane integrity and reduced growth or cell death. The data also demonstrated a considerable loss of cell viability, which was verified by superior Evans blue absorption. The data exposed could be the result of dopamine-induced oxidative stress, due to possible ROS produced during its decomposition and auto-oxidative transformation into melanin. We suggest that the decrease in cell viability (Fig. 5) and reduced root growth (Table 1) could also be related to increased levels of ROS in the roots, as a result of dopamine-induced oxidative stress due its conversion into melanin and due to its autoxidation. However, the possibility cannot be ruled out that there could be an increase in the level of auxin in roots treated with dopamine, contributing to the reduction in growth. Further studies are required to investigate dopamine metabolism in roots and to quantify auxin and ROS levels produced by this pathway. This is the purpose of a research currently in progress.
Materials and Methods
General procedures
Soybean [Glycine max (L.) Merr. cv BRS-232] seeds, surface-sterilized with 2% sodium hypochlorite for 5 min and rinsed extensively with deionized water, were dark-germinated (at 25°C) on three sheets of moistened filter paper. Twenty-five 3-d-old seedlings of uniform size were supported on an adjustable acrylic plate and transferred into a glass container (10 × 16 cm) filled with 200 ml of half-strength Hoagland solution (pH 6.0), without or with 0.25 to 1.0 mM dopamine. The container was kept in a growth chamber (25°C, 12L:12D photoperiod, irradiance of 280 μmol m−2 s−1). Roots were measured at the start and at the end of experiments (24 h). Fresh root weight was determined immediately after incubation and dry weight estimated after oven-drying at 80°C, for 24 h. Dopamine was purchased from Sigma Chemical Co. and all other reagents used were of the purest grade available or chromatographic grade.
Enzymatic assays
After incubation, all treated or untreated seedling roots were detached and enzymes were extracted. PAL was extracted, as described by Ferrarese et al.29 Fresh roots (2 g) were ground at 4°C in 0.1 M sodium borate buffer (pH 8.8). Homogenates were centrifuged (2,200g, 15 min) and the supernatant was used as the enzyme preparation. The reaction mixture (100 μmoles sodium borate buffer, pH 8.7 and a suitable amount of enzyme extract in a final volume of 1.5 ml) was incubated at 40°C, for 5 min, for PAL activity assay. Fifteen μmoles of l-phenylalanine were added to start the reaction which was stopped after 1 h of incubation by the addition of 50 μl 5 M HCl. Samples were filtered through a 0.45 μm disposable syringe filter and analyzed (20 μl) with a Shimadzu® Liquid Chromatograph equipped with a LC-10AD pump, a Rheodine® injector, a SPD-10A UV detector, a CBM-101 Communications Bus Module and a class-CR10 workstation system. A reversed-phase Shimpack® GLC-ODS (M) column (150 × 4.6 mm, 5 μm) was used at room temperature, with an equivalent pre-column (10 × 4.6 mm). The mobile phase was methanol:water (70%:30%) with a flow rate of 0.5 ml min−1. Absorption was measured at 275 nm. Data collection and integration were performed with class-CR10 software (Shimadzu®). t-Cinnamate, the product of PAL, was identified by comparing its retention time with that of standard’s. Parallel controls without l-phenylalanine or with t-cinnamate (added as internal standard in the reaction mixture) were made as described elsewhere.29 PAL activity was expressed as μmol t-cinnamate h−1 g−1 of fresh weight.
Cell wall-bound POD was extracted from fresh roots (0.5 g) with 67 mM phosphate buffer (5 ml, pH 7.0). Extract was centrifuged (2,200g, 5 min, 4°C) and the pellet was washed with deionized water until no soluble POD activity was detected in the supernatant. Pellet was then incubated in 1 M NaCl (2 ml, 1 h, 4°C) and the homogenate was centrifuged (2,200g, 5 min). The supernatant contained the cell wall (ionically) bound POD. Guaiacol-dependent activities of the cell wall-bound POD were determined according to Cakmak and Horst,30 with slight modifications. The reaction mixture (3 ml) contained 25 mM sodium phosphate buffer, pH 6.8, 2.58 mM guaiacol and 10 mM H2O2. Reaction started by adding the enzyme extract in phosphate buffer. Guaiacol oxidation was followed for 5 min, at 470 nm and enzyme activity was calculated from the extinction coefficient (25.5 mM−1 cm−1) for tetraguaiacol. Blank consisted of a reaction mixture without enzyme extract whose absorbance was subtracted from the mixture with enzyme extract. POD activities were expressed as μmol tetraguaiacol min−1 g−1 fresh weight.
SOD activity was assayed by using the photochemical nitroblue tetrazolium (NBT) method.31 Fresh roots (0.5 g) were ground in a mortar with 0.01 g of PVPP and 2 ml of 50 mM potassium phosphate buffer (pH 7.8) containing 1.0 mM EDTA. After centrifugation (2,200g, 20 min, 4°C), the supernatant was used for the determination of the enzyme activity. The reaction mixture (1.5 ml) contained 75 μM NBT, 4 μM riboflavin, 13 μM methionine, 50 mM phosphate buffer (pH 7.8) containing 1.0 mM EDTA and 20 μl of enzyme extract. To exclude eventual interference on SOD activity, parallel controls with dopamine added in the reaction mixture without enzyme preparation were undertaken under the same experimental conditions. The reaction was initiated by placing the reaction-mixture tubes under 15 W fluorescent lamps (56 μmol m−2 s−1) for 10 min. The reaction was stopped by keeping the tubes in the dark for 10 min. The photoreduction of NBT (formation of purple formazan) was measured at 560 nm. One unit of SOD enzyme activity was defined as the amount of enzyme required to produce a 50% inhibition of the reduction of NBT. SOD activity is expressed as unit g−1 fresh weight.
Lignin quantification
About 20 mg of protein-free cell wall from different tissue was weight into a screw capped glass tube and added 0.5 ml of 25% acetyl bromide (v/v in glacial acetic acid) as described by Hatfield.32 The mixture in the tube was allowed to react for 30 min at 70°C. After cooling in ice bath, 0.9 ml of 2 M NaOH, 0.1 ml of 5 M hydroxylamine-HCl and 2 ml of iced acetic acid were added to the samples and centrifuged (1,400g, 5 min). Blank was run in conjunction with the samples and the UV absorption spectrum measured against the blank at 280 nm. Standard curve was generated by commercial alkaline lignin (Aldrich) to the same procedure. The extinction coefficient was ε = 16.4 cm−1mg ml−1and lignin results were expressed as mg g−1 cell wall.
Cell viability
After incubation, the loss of cell viability in the seedlings was determined using the Evans blue staining spectrophotometric assay.33 All freshly harvested roots were incubated for 15 min with 30 ml of 0.25% Evans blue solution. Next, the roots were washed in distilled water for 30 min to remove excess and unbound dye and the excised root tips (3 cm) were soaked in 3 ml of N,N-dimethylformamide for 50 min at room temperature. The absorbance of released Evans blue solution was measured at 600 nm, using deionized water as a blank. The loss of cell viability is expressed as the absorbance at 600 nm of treated roots in relation to untreated roots (control).
Statistical design
The experimental design was completely randomized and each plot was represented by one glass container with 25 seedlings. Data are expressed as mean of three to six independent experiments ± SE. Significance of differences was undertaken by one-way variance analysis with GraphPad Prism® package (Version 2.0, GraphPad Software Inc.). Difference between parameters was evaluated by Dunnett’s multiple comparison test and p values < 0.05 were considered statistically significant.
Acknowledgements
Research was financially supported by the Brazilian Council for Scientific and Technological Development (CNPq). The authors kindly thank Aparecida M. D. Ramos and Fabiano Rodrigo de Assis for their technical assistance.
Glossary
Abbreviations:
- superoxide dismutase
SOD
- phenylalanine ammonia-lyase
PAL
- cell wall-bound peroxidase
POD
- L-3,4-dihydroxyphenylalanine
L-DOPA
- 3-indole acetic acid
IAA
- reactive oxygen species
ROS
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25477
References
- 1.Inderjit, Duke SO. Ecophysiological aspects of allelopathy. Planta. 2003;217:529–39. doi: 10.1007/s00425-003-1054-z. [DOI] [PubMed] [Google Scholar]
- 2.Weir TL, Park S-W, Vivanco JM. Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol. 2004;7:472–9. doi: 10.1016/j.pbi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Kulma A, Szopa J. Catecholamines are active compounds in plants. Plant Sci. 2007;172:433–40. doi: 10.1016/j.plantsci.2006.10.013. [DOI] [Google Scholar]
- 4.Anaya AL. Allelopathy as a tool in the management of biotic resources in agroecosystems. Crit Rev Plant Sci. 1999;18:697–739. doi: 10.1080/07352689991309450. [DOI] [Google Scholar]
- 5.Wichers HJ, Visser JF, Huizing HJ, Pras N. Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens and effects of 2,4-D and NaCI on these compounds. Plant Cell Tissue Organ Cult. 1993;33:259–64. doi: 10.1007/BF02319010. [DOI] [Google Scholar]
- 6.Matsumoto H. The mechanisms of phytotoxic action and selectivity of non-protein aromatic amino acids L-DOPA and m-tyrosine. J Pestic Sci. 2011;36:1–8. doi: 10.1584/jpestics.R10-15. [DOI] [Google Scholar]
- 7.Yue M-E, Jiang T-F, Shi Y-P. Simultaneous determination of noradrenaline and dopamine in Portulaca oleracea L. by capillary zone electrophoresis. J Sep Sci. 2005;28:360–4. doi: 10.1002/jssc.200400045. [DOI] [PubMed] [Google Scholar]
- 8.Kanazawa K, Sakakibara H. High content of dopamine, a strong antioxidant, in Cavendish banana. J Agric Food Chem. 2000;48:844–8. doi: 10.1021/jf9909860. [DOI] [PubMed] [Google Scholar]
- 9.Lundström J, Agurell S. Biosynthesis of mescaline and tetrahydroisoquinoline alkaloids in Lophophora williamsii (Lem.) Coult. Acta Pharm Suec. 1971;8:261–74. [PubMed] [Google Scholar]
- 10.Allen JF. Superoxide as an obligatory, catalytic intermediate in photosynthetic reduction of oxygen by adrenaline and dopamine. Antioxid Redox Signal. 2003;5:7–14. doi: 10.1089/152308603321223496. [DOI] [PubMed] [Google Scholar]
- 11.Van Alstyne KL, Nelson AV, Vyvyan JR, Cancilla DA. Dopamine functions as an antiherbivore defense in the temperate green alga Ulvaria obscura. Oecologia. 2006;148:304–11. doi: 10.1007/s00442-006-0378-3. [DOI] [PubMed] [Google Scholar]
- 12.Kuklin AI, Conger BV. Enhancement of somatic embryogenesis in orchardgrass leaf cultures by epinephrine. Plant Cell Rep. 1995;14:641–4. doi: 10.1007/BF00232729. [DOI] [PubMed] [Google Scholar]
- 13.Khurana JP, Tamot BK, Maheshwari N, Maheshwari SC. Role of catecholamines in promotion of flowering in a short-day duckweed, Lemna paucicostata 6746. Plant Physiol. 1987;85:10–2. doi: 10.1104/pp.85.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elstner EF, Konze JR, Selman BR, Stoffer C. Ethylene formation in sugar beet leaves: evidence for the involvement of 3-hydroxytyramine and phenoloxidase after wounding. Plant Physiol. 1976;58:163–8. doi: 10.1104/pp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klegeris A, Korkina LG, Greenfield SA. Autoxidation of dopamine: a comparison of luminescent and spectrophotometric detection in basic solutions. Free Radic Biol Med. 1995;18:215–22. doi: 10.1016/0891-5849(94)00141-6. [DOI] [PubMed] [Google Scholar]
- 16.Passardi F, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005;24:255–65. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- 17.Soares AR, Cássia Siqueira-Soares R, Salvador VH, Lourdes Lucio Ferrarese M, Ferrarese-Filho O. The effects of l-DOPA on root growth, lignification and enzyme activity in soybean seedlings. Acta Physiol Plant. 2012;34:1811–7. doi: 10.1007/s11738-012-0979-x. [DOI] [Google Scholar]
- 18.Devi SR, Prasad MNV. Ferulic acid mediated changes in oxidative enzymes of maize seedlings: implications in growth. Biol Plant. 1996;38:387–95. doi: 10.1007/BF02896668. [DOI] [Google Scholar]
- 19.Herrig V, Ferrarese MdeL, Suzuki LS, Rodrigues JD, Ferrarese-Filho O. Peroxidase and phenylalanine ammonia-lyase activities, phenolic acid contents, and allelochemicals-inhibited root growth of soybean. Biol Res. 2002;35:59–66. doi: 10.4067/S0716-97602002000100009. [DOI] [PubMed] [Google Scholar]
- 20.dos Santos WD, Ferrarese MdeL, Finger A, Teixeira ACN, Ferrarese-Filho O. Lignification and related enzymes in Glycine max root growth-inhibition by ferulic acid. J Chem Ecol. 2004;30:1203–12. doi: 10.1023/B:JOEC.0000030272.83794.f0. [DOI] [PubMed] [Google Scholar]
- 21.Protacio CM, Dai YR, Lewis EF, Flores HE. Growth stimulation by catecholamines in plant tissue/organ cultures. Plant Physiol. 1992;98:89–96. doi: 10.1104/pp.98.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taiz L, Zeiger E, eds. Fisiologia vegetal Porto Alegre: Artmed, 2009. [Google Scholar]
- 23.Kruk I, Lichszteld K, Bounias M, Kadna A, Kubera-Nowakowska L. Formation of active oxygen species during autoxidation of Dopa. Chemosphere. 1999;39:443–53. doi: 10.1016/S0045-6535(99)00007-7. [DOI] [Google Scholar]
- 24.Pattison DI, Dean RT, Davies MJ. Oxidation of DNA, proteins and lipids by DOPA, protein-bound DOPA, and related catechol(amine)s. Toxicology. 2002;177:23–37. doi: 10.1016/S0300-483X(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 25.Khaldy H, Escames G, León J, Vives F, Luna JD, Acuña-Castroviejo D. Comparative effects of melatonin, L-deprenyl, Trolox and ascorbate in the suppression of hydroxyl radical formation during dopamine autoxidation in vitro. J Pineal Res. 2000;29:100–7. doi: 10.1034/j.1600-079X.2000.290206.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosei MA, Blarzino C, Foppoli C, Mosca L, Coccia R. Lipoxygenase-catalyzed oxidation of catecholamines. Biochem Biophys Res Commun. 1994;200:344–50. doi: 10.1006/bbrc.1994.1454. [DOI] [PubMed] [Google Scholar]
- 27.Takano K, Tanaka N, Kawabe K, Moriyama M, Nakamura Y. Extracellular superoxide dismutase induced by dopamine in cultured astrocytes. Neurochem Res. 2013;38:32–41. doi: 10.1007/s11064-012-0882-2. [DOI] [PubMed] [Google Scholar]
- 28.Lai C-T, Yu PHR. R(-)-deprenyl potentiates dopamine-induced cytotoxicity toward catecholaminergic neuroblastoma SH-SY5Y cells. Toxicol Appl Pharmacol. 1997;142:186–91. doi: 10.1006/taap.1996.8011. [DOI] [PubMed] [Google Scholar]
- 29.Ferrarese MLL, Rodrigues JD, Ferrarese-Filho O. Phenylalanine ammonia-lyase activity in soybean roots extract measured by reverse-phase high performance liquid chromatography. Plant Biol. 2000;2:152–3. doi: 10.1055/s-2000-9162. [DOI] [Google Scholar]
- 30.Cakmak I, Horst W. Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83:463–8. doi: 10.1111/j.1399-3054.1991.tb00121.x. [DOI] [Google Scholar]
- 31.Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–14. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatfield RD, Grabber J, Ralph J, Brei K. Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: some cautionary notes. J Agric Food Chem. 1999;47:628–32. doi: 10.1021/jf9808776. [DOI] [PubMed] [Google Scholar]
- 33.Zanardo DIL, Lima RB, Ferrarese MLL, Bubna GA, Ferrarese-Filho O. Soybean root growth inhibition and ligniðcation induced by p-coumaric acid. Environ Exp Bot. 2009;66:25–30. doi: 10.1016/j.envexpbot.2008.12.014. [DOI] [Google Scholar]