Abstract
Sedentary plant-parasitic nematodes maintain a biotrophic relationship with their hosts over a period of several weeks and induce the differentiation of root cells into specialized feeding cells. Nematode effectors, which are synthesized in the esophageal glands and injected into the plant tissue through the syringe-like stylet, play a central role in these processes. Previous work on nematode effectors has shown that the apoplasm is targeted during invasion of the host while the cytoplasm is targeted during the induction and the maintenance of the feeding site. A large number of candidate effectors potentially secreted by the nematode into the plant tissues to promote infection have now been identified. This work has shown that the targeting and the role of effectors are more complex than previously thought. This review will not cover the prolific recent findings in nematode effector function but will instead focus on recent selected examples that illustrate the variety of plant cell compartments that effectors are addressed to in order reach their plant targets.
Keywords: effectors, giant-cell, plant defense, plant parasitic nematodes, syncytia
Plant parasitic nematodes are obligate parasites that cause billions of dollars of crop losses annually.1 Although different species infect a variety of plant organs most species are restricted to roots. The most intensively studied are the sedentary root endoparasites; the root-knot nematodes (RKN) and cyst nematodes (CN), which are the most damaging species worldwide. An intriguing aspect of RKN and CN infection is the ability of these obligate parasites to hijack plant cell fate and induce the re-differentiation of root cells, generally in the elongation zone, into specialized feeding cells, called giant-cells in the case of RKN and syncytia in the case of CN. These cells are enlarged, multinucleate and metabolically hyperactive cells that provide the nutrients required ensuring completion of the parasite life cycle.2 Although there are superficial similarities between the feeding structures induced by RKN and CN, they have entirely different ontogenies and are thought to have evolved independently. A remarkable feature of parasitism by RKN and CN is the ability of these parasites to maintain a long-lasting biotrophic interaction with the host (up to 6 weeks) that allows withdrawal of nutrients from the cytoplasm of living plant cells until the progeny are produced. Plant-endoparasitic nematode interactions therefore provide a fascinating model for the study of fundamental aspects of plant cell differentiation and plant defense suppression by the pathogen.
Our understanding of the molecular mechanisms that underlie plant-nematode interactions is expanding and we are beginning to decipher the means by which the pathogen manipulates host plant cells. Effector proteins, secreted into host tissues by the nematode, are one of the key components of the molecular dialog leading to successful infection. Effectors can be defined as the molecules secreted by the pathogen that alter host-cell physiology to promote infection in susceptible hosts.3 These effectors, or the modifications they induce in plant cell structures or functions, can be perceived by the plant thus triggering defense responses.3 Nematode effectors can be secreted from the hypodermis on to the cuticle surface or can be produced in specialized secretory organs. Effectors secreted through the stylet are the most studied in RKN and CN.4,5 These effectors are produced by unicellular esophageal glands, secreted into the esophagus by exocytosis and delivered into the plant tissue through the stylet which acts as a syringe and injects these esophageal secretions into the host tissue. The stylet is also used to withdraw nutrients from the feeding cell cytoplasm.
The recent proliferation of reviews on nematode effectors demonstrates the dynamism of this field of research. These reviews highlight the sophisticated means developed by nematodes to manipulate plant cell physiology and plant defenses.4-10 This review will not cover the prolific recent findings in nematode effector functions but will instead focus on recent examples that illustrate the variety of plant cell compartments that effectors are addressed to in order to reach their plant targets.
Apoplasm
Cell wall modifying and degrading enzymes such as cellulases and pectate lyases were the first nematode secreted proteins to be localized in planta during infection. These enzymes are produced in the nematode subventral gland cells and have been immuno-localized at the stylet orifice of migrating juveniles and along the migratory path of the CN Heterodera glycines and the RKN Meloidogyne javanica and M. incognita. These studies, in combination with work confirming the biochemical activity of the enzymes, provide convincing evidence for a role in disruption of the plant cell wall during root invasion.11-13 An improved immuno-cytochemical method has recently been developed that preserves both the plant and the pathogen tissues; this method has subsequently been used to show that more proteins than previously thought are secreted into the apoplasm during infection.12 In addition, new roles for effectors secreted into the apoplasm are emerging. For example, a RKN calreticulin (Mi-CRT) was shown to be abundantly secreted into the apoplasm by M. incognita sedentary stages during induction and maintenance of the giant cells.14 The role of Mi-CRT as a suppressor of host innate immunity was recently demonstrated15 but its mode of action is still to be determined. The venom allergen-like effector Gr-VAP1 from the CN Globodera rostochiensis was shown to interact with an allelic form of the apoplastic papain-like cysteine protease Rcr3 of tomato and perturb its active site, thereby increasing plant susceptibility to the nematode.16 However, in resistant tomato plants this modification of Rcr3 is perceived by the transmembrane Cf2 receptor which triggers a hypersensitive response.16 Interestingly, the same allelic form of Rcr3 is also a virulence target for the leaf mold fungus Cladosporium fulvum, providing the first evidence for a common target for a nematode and a fungus pathogen and showing that plants have acquired defense mechanisms able to respond several pathogens during evolution.16
Cytoplasm
After migration is complete, the nematode perforates the cell wall of selected plant cells with its stylet to form the feeding site. Electron micrographs have clearly shown that the stylet comes into contact with the plasma membrane. An aperture is formed and dense materials subsequently accumulate in the plant cytoplasm, suggesting that some stylet secretions are injected directly into the cytoplasm of the feeding cells.17 In this regard, the nematode stylet may in some ways be considered as functionally analogous to the type II or type III secretion systems of pathogenic bacteria that inject directly effectors into host cells.18 However, the direct observation of nematode secretions in the plant cell cytoplasm is technically challenging due to the low quantity of nematode secreted proteins present in the enlarged feeding cells. In addition, the inability to transform plant parasitic nematodes makes it impossible to use reporter fusions to trace nematode secretions in vivo. However, several lines of evidence indicate that some stylet secretions are targeted to the plant cell cytoplasm, including the heterologous expression in plants of effector-reporter fusion proteins or the identification of candidate plant targets that are localized in the cytoplasm. Of course, such indirect evidence needs to be considered with circumspection. For example, protein overexpression might lead to accumulation of secretory material in the cytoplasm that does not reflect the actual destination of proteins secreted by the nematode. In addition, the yeast two hybrid systems generally used to identify effector targets are optimized for cytoplasmic proteins. However, much effort is put into to confirming these results using other tools such as protein-protein interaction assays in vitro or in planta and confirmation that the candidate target proteins are actually expressed in infected plant tissues. In the case of the Hs4F01 annexin-like effector from Heterodera schachtii, a proposed role for this effector in the cytoplasm was confirmed by the complementation of AnnAt1 Arabidopsis annexin mutants, showing that Hs4F01 is able mimic host annexin function in plant cells to regulate stress responses during infection.19 Several plant targets identified for nematode effectors and located in the cytoplasm are related to plant defense. The Hs10A06 effector of H. schachtii interacts with plant spermidine synthase in the cytoplasm in order to modulate salicylic acid signaling and antioxidant machinery, thereby modulating the plant cell defense response.20 The CN G. pallida and G. rostochiensis secrete so-called SPRYSEC proteins that contain a SPRYmotif is their peptide sequence. Some of these effectors are able to interact with plant NB-LRR-resistance proteins in the cytoplasm. For example, the effector SPRYSEC-19 of G. rostochiensis interacts with the LRR domain of SW5F, a member of the tomato SW5 gene cluster that contains several resistance genes to pathogens.21 Surprisingly, co-expression of SPRYSEC-19 and SW5F in tobacco or tomato leaves did not trigger a defense-related programmed cell death. Conversely, the SPRYSEC effector GpRBP1 from G. pallida induced programmed cell death when co-expressed with the CC-NB-LRR nematode resistance protein Gpa2 from potato in Nicotiana benthamiana leaves.22 The complexity of the interplay between SPRYSEC effectors and plant resistance genes was further highlighted when SPRYSEC-19 was shown to suppress Gpa2-GpRBP1 mediated programmed cell death.23 In some cases, the nematode effectors play a role in the cytoplasm in the region of the plasma membrane. Co-localization studies in vivo by bimolecular fluorescence complementation have shown that the effector Hs19C07, which is secreted by the CN H. schachtii, interacts with the transmembrane auxin influx transporter LAX3 at the plasma membrane,24 most probably to manipulate auxin influx and allow the differentiation of the feeding cell as previously anticipated.25
Trafficking between Apoplasm and Cytoplasm
As afore mentioned, electron micrograph images suggest that the nematode is capable of secreting effectors directly into the host cytoplasm and show the stylet orifice in contact with the host plasma membrane, rather than being deeply inserted into the host cytoplasm. In addition, nematodes clearly access the cytoplasm during feeding. On the other hand, a number of nematode effectors have been localized in the apoplasm of infected plants, raising the possibility that nematode effectors could be translocated from the apoplasm to the cytoplasm of plant cells. Some pathogen effectors, notably effectors from oomycetes and fungi, are first secreted by the pathogen and subsequently translocated into the cytoplasm of infected cells.3 In oomycetes, translocation is mediated by conserved RXLR and LXLFLAK motifs, although the precise mechanism by which this occurs is still a matter of debate.26,27 In fungi, translocation motifs have been identified that show no conservation in sequence or structure.28Yet, no functional evidence for the translocation of nematode effectors from the apoplasm to the cytoplasm has been found. A search for conserved motifs in nematode effectorsthat took into consideration the physico-chemical properties of amino-acids identified several potential motifs that were discriminative of effectors as compared with secreted proteins unrelated to parasitism. The motif L L I I S and several variants with similar physico-chemical properties, including length variants, were highly represented in effectors but absent from control proteins.29 These motifs are located within the 30 first amino acids at the N-terminal of the proteins, but systematically absent from the signal peptides of control proteins. It is feasible that they could be related to effector secretion from the nematode and/or targeting to the final plant cell compartment.
Strikingly, several studies have shown the remarkable ability of some nematode effectors to traffic from the cytoplasm to the apoplasm. The CLE-like peptides secreted by the CN H. glycines share similarity with CLAVATA3 (CLV3)/ESR hormon peptides that control the fate of meristematic cells in plants. Several lines of evidence including complementation and infection assays on plants deficient for CLE signaling have shown that the nematode CLE-like effectors target plant receptors expressed in syncytia to mimic CLV3 signaling.30 During infection the CLE-like effectors are first introduced into the cytoplasm before being re-directed to the apoplasm. This secretion is independent of the plant cell ER secretory pathway and is mediated by a variable region of the protein immediately upstream of the CLE domain.31 It has been suggested that the nematode CLE-like effectors interact with plant cell receptors to re-direct signaling pathways active in roots and trigger developmental cascades required for feeding cell differentiation.30 A second example of a nematode effector being re-directed from the cytoplasm to the apoplasm was provided by the nematode effector Hs CBP which is similar to Cellulose Binding Proteins (CBP) and targets the pectin methylesterase protein 3 (PME3) in Arabidopsis thaliana. However, it is not yet known whether PME3 can act as a carrier for Hs CBP in order to mediate its export from the host plant cytoplasm to the apoplast and the cell wall.9
Nucleus
Many in the plant-nematode community investigate subcellular localization of effectors using heterologous expression inside plant cells, particularly transient expression in the model plant N. benthamiana.32-34 The first direct evidence for nuclear targeting of a nematode effector was obtained by immunolocalization of effector Mi-EFF1 from M. incognita on sections of infected tomato roots.33 As is the case for other plant-pathogen interactions, the targeting of plant cell nucleus by effectors suggests that manipulation of host transcription is an important strategy developed by the nematode to counteract plant defense responses. Plant nematodes will also need to induce changes in host gene expression in order to induce the formation of a feeding structure and nuclear effectors may be important for this process. Effectors may manipulate host transcription or may directly target essential nuclear host components for the benefit of the pathogen. For example, the RKN effector 16D10 has been shown to interact with putative SCARECROW transcription factors.35
Trafficking from Cytoplasm to Nucleus
Ten years ago, an ubiquitin extension protein (Hs-UBI1) was identified as a H. schachtii effector that is composed of an N-terminal signal peptide, a ubiquitin domain and a 22 aa C-terminal extension protein with no similarity to any known protein. Heterologous expression in tobacco cells suggested that after secretion from the dorsal esophageal gland of the nematode and injection into the plant cell cytoplasm, the C-terminal domain was cleaved and targeted to the nucleolus.36 More recently, it was shown by immunolocalization that a ubiquitin carboxyl extension protein (GrUBCEP12) is similarly secreted from the dorsal esophageal gland in G. rostochiensis.37 Transient expression studies in N.benthamiana leaves showed that the effector is processed in the cytoplasm into free ubiquitin and a 12 aa CEP12 peptide. Fusion constructs that carried the CEP12 peptide were localized in the cytoplasm and in the nucleus of transfected plant cells. Interestingly, additional functional assays in planta showed that the ubiquitin domain of GrUBCEP12 seemed to control the 26S proteasome activity by regulating the expression of a 26S subunit whereas GrCEP12 suppressed plant cell death triggered by the Gpa2/RBP–1 and Rx2/CP resistance/avirulence proteins.37 The mode of action of the CEP12 peptide and whether it has any activity in the nucleus, are still to be determined. Another CN effector, Hs32E03, is located in the nucleus following transient expression in plant cells.32 Studies are underway to identify candidate targets for this effector and these suggest that the effector binds to a cytoplasmic protein before trafficking to the nucleus.9
Conclusions
Expanding information on the identification and functional analysis of nematode effectors have shown a wide variety of proteins secreted and the variety of their plant targets. It is now widely accepted that nematode effectors, in particular stylet secretions, play key roles in the manipulation of plant physiology that allow the nematode to get the most from its host. To promote parasite development, these effectors may participate in the formation of feeding structures and the suppression of plant defenses. The diversity in nematode effector functions is highlighted by the diversity of their destination sub-cellular compartments in plant cells (sum-up in Fig. 1). These findings have raised new appealing questions, such as understanding how nematode effectors transit between plant cell compartments to reach their respective targets and succeed in fine tuningof plant physiology networks.
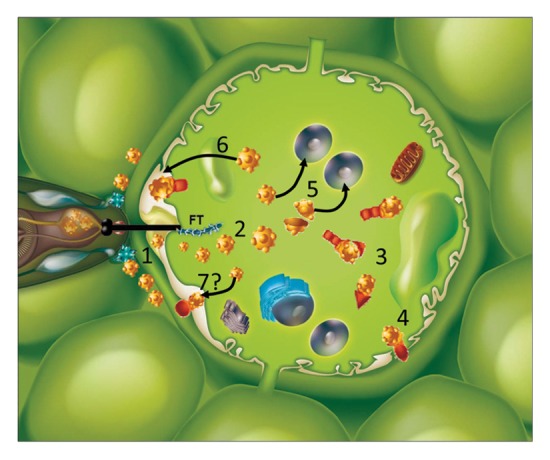
Figure 1. Nematode effectors are targeted to a variety of plant cell compartments. Effectors secreted in the apoplasm have been identified that are involved in plant cell wall disruption during root invasion, increased virulence or increased plant susceptibility. Effectors injected into the cytoplasm (2) have a variety of plant targets (3), including trans-membrane proteins (4). They can be directed to the nucleus (5), or the apoplasm (6).Whether some effectors are re-directed to the plant wall is still to be determined (7).
Acknowledgments
Research from Maëlle Jaouannet and Marie-Noëlle Rosso on nematode effectors was done at INRA, UMR 1355, CNRS, UMR 7254, Université de Nice Sophia Antipolis, Institut Sophia Agrobiotech, F-06903 Sophia Antipolis, France
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25507
References
- 1.Blok VC, Jones JT, Phillips MS, Trudgill DL. Parasitism genes and host range disparities in biotrophic nematodes: the conundrum of polyphagy versus specialisation. Bioessays. 2008;30:249–59. doi: 10.1002/bies.20717. [DOI] [PubMed] [Google Scholar]
- 2.Hussey RS, Grundler FM. Nematode parasitism of plants. In: Perry RN, Wright DJ, eds. The physiology and biochemistry of free-living and plant-parasitic nematodes. Wallingford, Oxon, UK: CABI Pub., 1998:213-43. [Google Scholar]
- 3.Hogenhout SA, Van der Hoorn RAL, Terauchi R, Kamoun S. Emerging concepts in effector biology of plant-associated organisms. Mol Plant Microbe Interact. 2009;22:115–22. doi: 10.1094/MPMI-22-2-0115. [DOI] [PubMed] [Google Scholar]
- 4.Haegeman A, Mantelin S, Jones JT, Gheysen G. Functional roles of effectors of plant-parasitic nematodes. Gene. 2012;492:19–31. doi: 10.1016/j.gene.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Rosso M-N, Hussey RS, Davis EL, Smant G, Baum TJ, Abad P, et al. Nematode Effector Proteins: Targets and Functions in Plant Parasitism. In: Martin F, Kamoun S, eds. Effectors in Plant–Microbe Interactions: Wiley-Blackwell, 2012:327-54. [Google Scholar]
- 6.Bellafiore S, Briggs SP. Nematode effectors and plant responses to infection. Curr Opin Plant Biol. 2010;13:442–8. doi: 10.1016/j.pbi.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Davis EL, Hussey RS, Baum TJ. Getting to the roots of parasitism by nematodes. Trends Parasitol. 2004;20:134–41. doi: 10.1016/j.pt.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Gheysen G, Mitchum MG. How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol. 2011;14:415–21. doi: 10.1016/j.pbi.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Hewezi T, Baum TJ. Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant Microbe Interact. 2013;26:9–16. doi: 10.1094/MPMI-05-12-0106-FI. [DOI] [PubMed] [Google Scholar]
- 10.Quentin M, Abad P, Favery B. Plant parasitic nematode effectors target host defence and nuclear functions to establish feeding cells. Front Plant Sci. 2013;4:1–7. doi: 10.3389/fpls.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle EA, Lambert KN. Cloning and characterization of an esophageal-gland-specific pectate lyase from the root-knot nematode Meloidogyne javanica. Mol Plant Microbe Interact. 2002;15:549–56. doi: 10.1094/MPMI.2002.15.6.549. [DOI] [PubMed] [Google Scholar]
- 12.Vieira P, Danchin EGJ, Neveu C, Crozat C, Jaubert S, Hussey RS, et al. The plant apoplasm is an important recipient compartment for nematode secreted proteins. J Exp Bot. 2011;62:1241–53. doi: 10.1093/jxb/erq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Meyers D, Yan Y, Baum T, Smant G, Hussey R, et al. In planta localization of a beta-1,4-endoglucanase secreted by Heterodera glycines. Mol Plant Microbe Interact. 1999;12:64–7. doi: 10.1094/MPMI.1999.12.1.64. [DOI] [PubMed] [Google Scholar]
- 14.Jaubert S, Milac AL, Petrescu AJ, de Almeida-Engler J, Abad P, Rosso M-N. In planta secretion of a calreticulin by migratory and sedentary stages of root-knot nematode. Mol Plant Microbe Interact. 2005;18:1277–84. doi: 10.1094/MPMI-18-1277. [DOI] [PubMed] [Google Scholar]
- 15.Jaouannet M, Magliano M, Arguel MJ, Gourgues M, Evangelisti E, Abad P, et al. The root-knot nematode calreticulin Mi-CRT is a key effector in plant defense suppression. Mol Plant Microbe Interact. 2013;26:97–105. doi: 10.1094/MPMI-05-12-0130-R. [DOI] [PubMed] [Google Scholar]
- 16.Lozano-Torres JL, Wilbers RH, Gawronski P, Boshoven JC, Finkers-Tomczak A, Cordewener JH, et al. Dual disease resistance mediated by the immune receptor Cf-2 in tomato requires a common virulence target of a fungus and a nematode. Proc Natl Acad Sci USA. 2012;109:10119–24. doi: 10.1073/pnas.1202867109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobczak M, Golinowski W, Grundler F. Ultrastructure of feeding plugs and feeding tubes formed by Heterodera schachtii. Nematology. 1999;1:363–74. doi: 10.1163/156854199508351. [DOI] [Google Scholar]
- 18.Torto-Alalibo T, Collmer CW, Gwinn-Giglio M, Lindeberg M, Meng S, Chibucos MC, et al. Unifying themes in microbial associations with animal and plant hosts described using the gene ontology. Microbiol Mol Biol Rev. 2010;74:479–503. doi: 10.1128/MMBR.00017-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel N, Hamamouch N, Li C, Hewezi T, Hussey RS, Baum TJ, et al. A nematode effector protein similar to annexins in host plants. J Exp Bot. 2010;61:235–48. doi: 10.1093/jxb/erp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewezi T, Howe PJ, Maier TR, Hussey RS, Mitchum MG, Davis EL, et al. Arabidopsis spermidine synthase is targeted by an effector protein of the cyst nematode Heterodera schachtii. Plant Physiol. 2010;152:968–84. doi: 10.1104/pp.109.150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehman S, Postma W, Tytgat T, Prins P, Qin L, Overmars H, et al. A secreted SPRY domain-containing protein (SPRYSEC) from the plant-parasitic nematode Globodera rostochiensis interacts with a CC-NB-LRR protein from a susceptible tomato. Mol Plant Microbe Interact. 2009;22:330–40. doi: 10.1094/MPMI-22-3-0330. [DOI] [PubMed] [Google Scholar]
- 22.Sacco MA, Koropacka K, Grenier E, Jaubert MJ, Blanchard A, Goverse A, et al. The cyst nematode SPRYSEC protein RBP-1 elicits Gpa2- and RanGAP2-dependent plant cell death. PLoS Pathog. 2009;5:e1000564. doi: 10.1371/journal.ppat.1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postma WJ, Slootweg EJ, Rehman S, Finkers-Tomczak A, Tytgat TOG, van Gelderen K, et al. The effector SPRYSEC-19 of Globodera rostochiensis suppresses CC-NB-LRR-mediated disease resistance in plants. Plant Physiol. 2012;160:944–54. doi: 10.1104/pp.112.200188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee C, Chronis D, Kenning C, Peret B, Hewezi T, Davis EL, et al. The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development. Plant Physiol. 2011;155:866–80. doi: 10.1104/pp.110.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunewald W, Cannoot B, Friml J, Gheysen G. Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog. 2009;5:e1000266. doi: 10.1371/journal.ppat.1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyler BM, Kale SD, Wang Q, Tao K, Clark HR, Drews K, et al. Microbe-independent entry of oomycete RxLR effectors and fungal RxLR-like effectors into plant and animal cells is specific and reproducible. Mol Plant Microbe Interact. 2013;26:611–6. doi: 10.1094/MPMI-02-13-0051-IA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wawra S, Djamei A, Albert I, Nürnberger T, Kahmann R, van West P. In vitro translocation experiments with RxLR-reporter fusion proteins of Avr1b from Phytophthora sojae and AVR3a from Phytophthora infestans fail to demonstrate specific autonomous uptake in plant and animal cells. Mol Plant Microbe Interact. 2013;26:528–36. doi: 10.1094/MPMI-08-12-0200-R. [DOI] [PubMed] [Google Scholar]
- 28.Ribot C, Césari S, Abidi I, Chalvon V, Bournaud C, Vallet J, et al. The Magnaporthe oryzae effector AVR1-CO39 is translocated into rice cells independently of a fungal-derived machinery. Plant J. 2013;74:1–12. doi: 10.1111/tpj.12099. [DOI] [PubMed] [Google Scholar]
- 29.Vens C, Rosso MN, Danchin EG. Identifying discriminative classification-based motifs in biological sequences. Bioinformatics. 2011;27:1231–8. doi: 10.1093/bioinformatics/btr110. [DOI] [PubMed] [Google Scholar]
- 30.Replogle A, Wang J, Paolillo V, Smeda J, Kinoshita A, Durbak A, et al. Synergistic interaction of CLAVATA1, CLAVATA2, and RECEPTOR-LIKE PROTEIN KINASE 2 in cyst nematode parasitism of Arabidopsis. Mol Plant Microbe Interact. 2013;26:87–96. doi: 10.1094/MPMI-05-12-0118-FI. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Lee C, Replogle A, Joshi S, Korkin D, Hussey R, et al. Dual roles for the variable domain in protein trafficking and host-specific recognition of Heterodera glycines CLE effector proteins. New Phytol. 2010;187:1003–17. doi: 10.1111/j.1469-8137.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- 32.Elling AA, Davis EL, Hussey RS, Baum TJ. Active uptake of cyst nematode parasitism proteins into the plant cell nucleus. Int J Parasitol. 2007;37:1269–79. doi: 10.1016/j.ijpara.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Jaouannet M, Perfus-Barbeoch L, Deleury E, Magliano M, Engler G, Vieira P, et al. A root-knot nematode-secreted protein is injected into giant cells and targeted to the nuclei. New Phytol. 2012;194:924–31. doi: 10.1111/j.1469-8137.2012.04164.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones JT, Kumar A, Pylypenko LA, Thirugnanasambandam A, Castelli L, Chapman S, et al. Identification and functional characterization of effectors in expressed sequence tags from various life cycle stages of the potato cyst nematode Globodera pallida. Mol Plant Pathol. 2009;10:815–28. doi: 10.1111/j.1364-3703.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang G, Dong R, Allen R, Davis EL, Baum TJ, Hussey RS. A root-knot nematode secretory peptide functions as a ligand for a plant transcription factor. Mol Plant Microbe Interact. 2006;19:463–70. doi: 10.1094/MPMI-19-0463. [DOI] [PubMed] [Google Scholar]
- 36.Tytgat T, Vanholme B, De Meutter J, Claeys M, Couvreur M, Vanhoutte I, et al. A new class of ubiquitin extension proteins secreted by the dorsal pharyngeal gland in plant parasitic cyst nematodes. Mol Plant Microbe Interact. 2004;17:846–52. doi: 10.1094/MPMI.2004.17.8.846. [DOI] [PubMed] [Google Scholar]
- 37.Chronis D, Chen S, Lu S, Hewezi T, Carpenter SCD, Loria R, et al. A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant J. 2013;74:185–96. doi: 10.1111/tpj.12125. [DOI] [PubMed] [Google Scholar]


