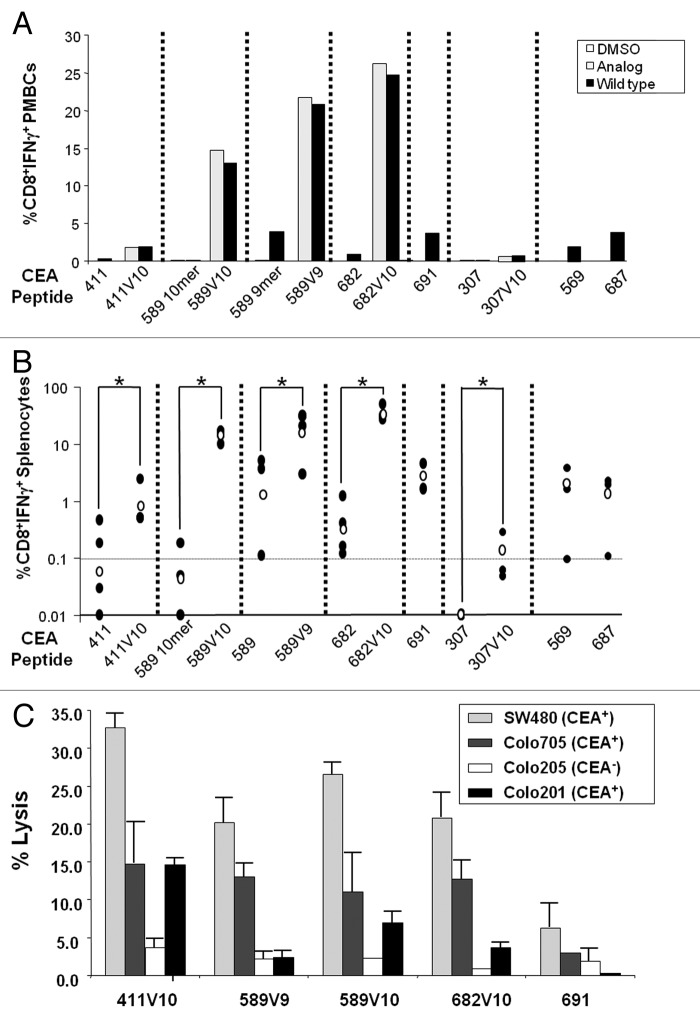
Figure 2. Immunogenicity of selected epitopes. (A–C) HHD mice were immunized with the indicated carcinoembryonic antigen (CEA) peptides (shown on the y-axis) by subcutaneous injection of 100 μg of the wild-type peptide or analog in a mixture with 140 μg HBV-T helper epitope58 and 50 μg CpG-ODN in Incomplete Freund Adjuvant (IFA). (A) Peripheral blood mononuclear cells (PBMCs) from immunized HHD mice were stimulated in vitro with the indicated peptide and analyzed for CD8+ T cell interferon-γ (IFNγ) secretion by intracellular staining and flow cytometry 2 to 3 wk after the last treatment. Mice immunized with analogs were analyzed both for cell-mediated immunity (CMI) elicited against the analog itself (black bar) and against the corresponding wild-type epitope (lightly shaded bar). Each sample was obtained by pooling PBMCs from 4–5 HHD mice and analyzed in duplicate. (B) Splenocytes from peptide immunized HHD mice were stimulated in vitro with pooled peptides and analyzed for CD8+ T cell IFNγ secretion by intracellular staining of cell-surface stained splenocytes and flow cytometry. Each black dot represents a single mouse; the empty dot represents the geometric mean of the group. The dashed horizontal lines represents a cut-off of 0.1% CD8+IFNγ+ cells chosen to indicate biologically relevant immunogenicity. Statistical analysis was performed by Student's t test; *P-value < 0.05. (C) Cytotoxic T lymphocyte (CTL) effectors isolated from spleens of immunized HHD mice (n = 3 per group) 5 d after final vaccination with the indicated CEA peptides were stimulated in vitro with peptide pools and recombinant human IL-2. On day 5, these in vitro stimulated cells were used as CTL effector cells, and the CTL activity was determined by a 6 h 51Cr-release cytotoxicity assay using the indicated cell lines as targets. Effector (E) cells were incubated with target (T) cells at E:T ratio = 100. Each bar represents the lysis obtained with effectors from each group vaccinated with the indicated peptide. Colo205 (CEA−) human colon adenocarcinoma cells served as a negative control. The assay was run in triplicate.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.