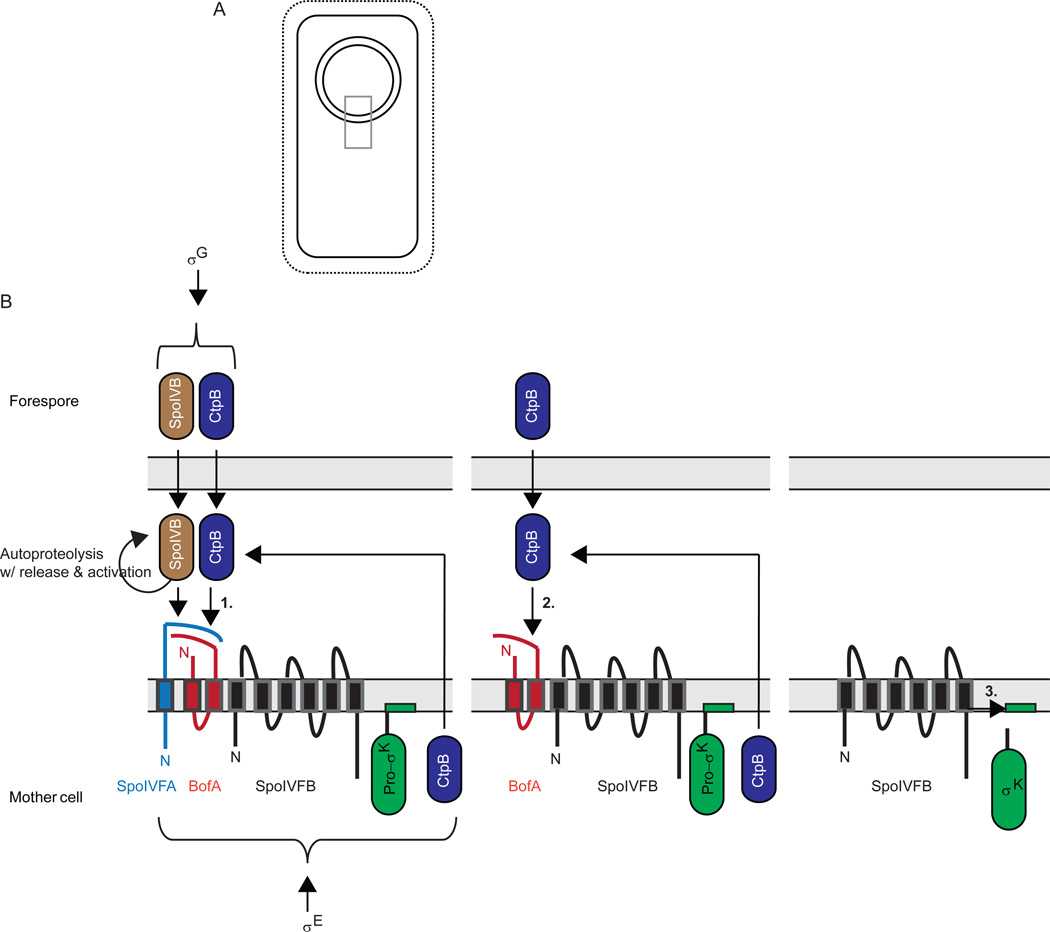
Figure 6. Activation of σK during B. subtilis sporulation.
(A) Upon completion of engulfment, the forespore is surrounded by two membranes. Grey box indicates region expanded in (B).
(B) Expanded view depicting a series of proteolytic cleavages (see text for references). First, σG in the forespore causes expression of serine proteases SpoIVB and CtpB (also expressed under σE control in the mother cell), which are translocated into the intermembrane space, where they cleave the C-terminal domain of SpoIVFA to initiate its degradation (1). SpoIVFA was in a complex with BofA and SpoIVFB in the outer membrane surrounding the forespore after these proteins were expressed in the mother cell under σE control. In a second step, CtpB and one or more other proteases (not shown) cleave BofA to initiate its degradation (2). Finally, SpoIVFB cleaves Pro-σK, releasing σK into the mother cell (3). The pro-sequence of Pro-σK is depicted to loop into the membrane based on findings that Pro-σK associates peripherally with membranes in B. subtilis (Zhang et al., 1998) or when expressed in E. coli (Zhou et al., 2013).