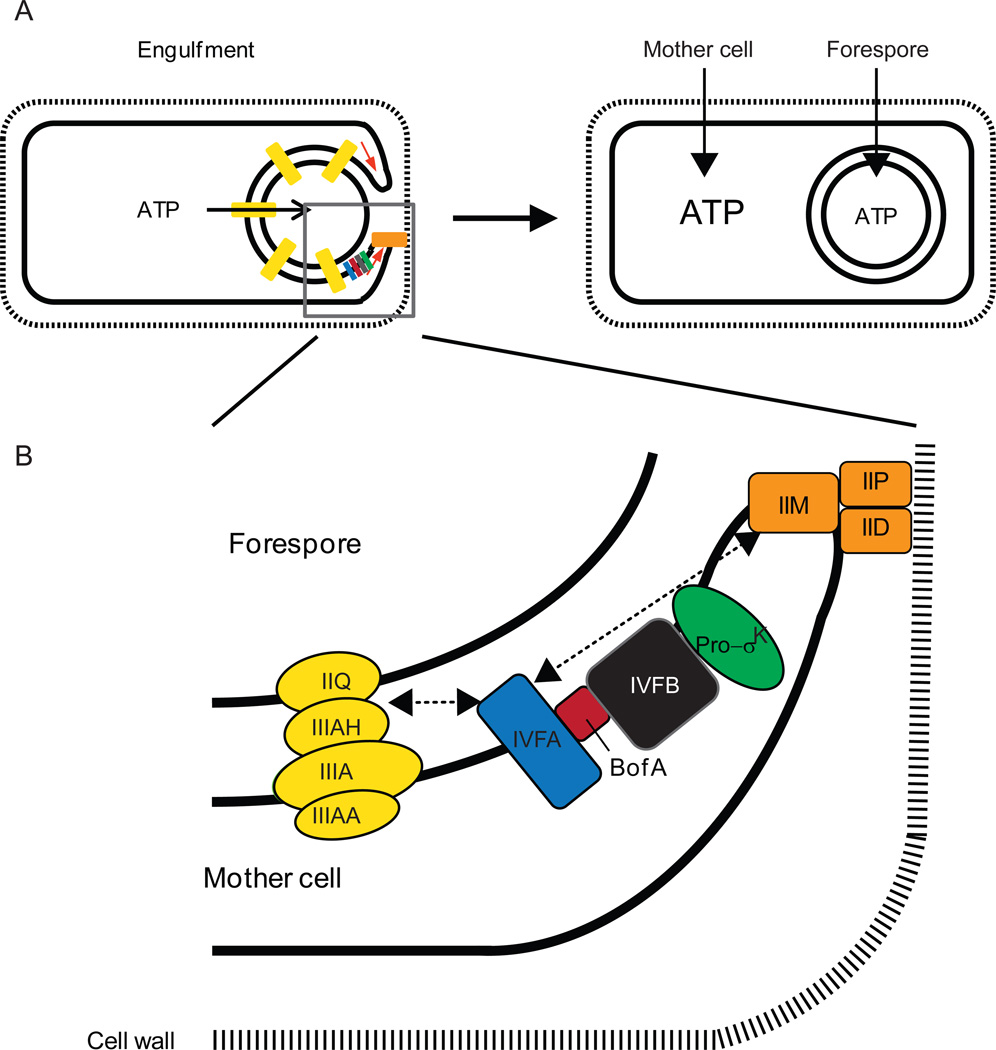
Figure 7. A model for ATP transport and accumulation during B. subtilis sporulation, and localization of the SpoIVFA-BofA-SpoIVFB complex with channel and engulfment complexes.
(A) A complex of proteins (orange) interacts with the cell wall and causes the mother cell membrane to engulf the forespore (left). During engulfment, channels (yellow) are formed that span the intermembrane space and have been proposed to allow small molecules like ATP to move from the mother cell into the forespore (see text for references). Upon completion of engulfment, the channels undergo reorganization and some components are degraded, perhaps allowing the ATP concentration to rise in the mother cell (right). ATP binding to the CBS domain of SpoIVFB would activate it to cleave Pro-σK, provided SpoIVFA and BofA have been degraded (Fig. 6). Grey box indicates region expanded in (B).
(B) Enlarged view of protein complexes during engulfment. SpoIVFA facilitates assembly of SpoIVFB with its inhibitor BofA and localizes the complex to foci that include the channel (yellow) and engulfment complexes (orange), although whether SpoIVFA interacts directly with a protein(s) in the other complexes or interacts indirectly is unknown (dashed arrows). Reprinted from (Kroos & Akiyama, 2013) with permission.