Abstract
Aim:
To study the pharmacologic effect of ZK14, a novel nitric oxide-donating biphenyldicarboxylate (DDB) derivative, on HSC-T6 cells and on CCl4-induced hepatic fibrosis.
Methods:
Inhibition of HSC-T6 cell growth by ZK14 was evaluated by MTT assay. The effect of ZK14 on the percentage of HSC-T6 cells undergoing apoptosis was measured using Annexin-V/PI double-staining and TUNEL assay. Mitochondrial membrane potential (MMP) and caspase activities were tested. Hepatic fibrosis was induced in Sprague-Dawley rats by intraperitoneal injection with 14% CCl4. Rats with hepatic fibrosis were randomly divided into four groups: model control, ZK14 (20 mg/kg), ZK14 (10 mg/kg) and DDB (5 mg/kg). Levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), hyaluronic acid (HA), type III collagen (PCIII), and nitric oxide (NO) were assessed, and liver samples were stained with hematoxylin-eosin. The NO level in cells treated with ZK14 in vitro was also measured.
Results:
The effect of ZK14 on HSC-T6 cell apoptosis was concentration- and time-dependent, with up to 50% of cells becoming apoptotic when exposed to 100 μmol/L ZK14 for 18 h. ZK14 treatment resulted in mitochondrial membrane depolarization and activation of caspases 3 and 9. At a dose of 20 mg/kg, ZK14 significantly decreased serum transaminase (AST, ALT) activities and fibrotic index (HA, PCIII) levels and significantly inhibited fibrogenesis.
Conclusion:
These data indicate that ZK14, a novel NO-donating DDB derivative, promotes HSC-T6 apoptosis in vitro through a signaling mechanism involving mitochondria and caspase activation and it inhibits CCl4-induced hepatic fibrosis in vivo. The results suggest that ZK14 has potential therapeutic value in the treatment of hepatic fibrosis.
Keywords: nitric oxide, apoptosis, hepatic fibrosis, HSC-T6, CCl4, biphenyldicarboxylate, ZK14
Introduction
Hepatic fibrosis is a dynamic process resulting from chronic liver injury of diverse etiology (viral, toxic, metabolic, autoimmune) and eventually leads to cirrhosis1. During such chronic injury, the perisinusoidal retinoid-storing quiescent hepatic stellate cells (HSCs) transform into retinoid-free proliferating myofibroblast-like cells, which give rise to subpopulations of stellate cells with discrete cytoskeletal and phenotypic profiles and thereby promote a net increase in extracellular matrix2.
Attention is increasingly focused on the mechanism by which liver fibrosis regresses, and in particular on the fate of activated stellate cells as fibrosis recedes3. Liver tissue can undergo regression of fibrosis after withdrawal of the damaging stimulus4, even at advanced stages of cirrhosis5. Gliotoxin, a fungal toxin, can provoke selective apoptosis in HSCs in culture and in vivo, leading to reduced fibrosis6, 7. However, the clinical usefulness of apoptosis-inducing drugs is limited due to the lack of cell specificity and the risk of severe adverse effects8, 9. Thus, although selective induction of apoptosis in HSCs is desirable, it is also important to find drugs that can prevent severe adverse effects such as further loss of liver function during progression of disease.
Biphenyldicarboxylate (DDB), a traditional therapeutic in the treatment of hepatic disease, is an intermediate in the synthesis of schizandrin. It can reduce the activity of glutamic-pyruvic transaminase in serum, is targeted to the liver and can protect injured hepatocytes and increase liver detoxifying function10, 11. DDB showed a better hepatoprotective effect than ursodesoxycholic acid and it has been suggested that it might prove of benefit in the therapy of chronic liver disease12, 13.
With these considerations in mind, we conjugated DDB to the nitric oxide (NO) donor furoxan, using amino acids as spacers in compound synthesis. We hypothesized that NO-releasing derivatives of DDB might have dual functions, inducing apoptosis in HSC-T6 cells by means of releasing NO and causing protection in hepatocytes by DDB.
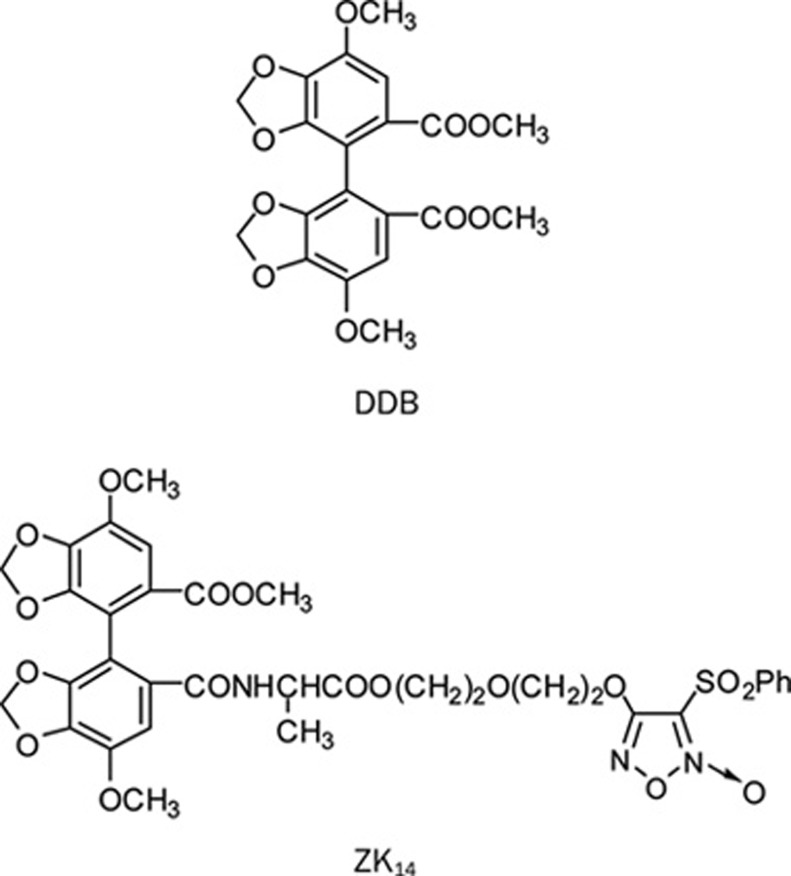
Pharmacological analysis of 55 NO-donating DDB derivatives revealed that 4,4′-dimethoxy-5,6,5′,6′-dimethylenedioxy-2-methoxycarbonyl-2′-{N-[alanine 2-(3-phenylsulfonyl-1,2,5-oxadiazole-2-oxide-4-) oxyethoxyethyl ester]} carbamoyl biphenyl (ZK14, Figure 1) had the strongest apoptosis-inducing activities in vitro14. It was therefore selected as the target compound. This study investigates the biological activity and mechanism of action of ZK14 in HSC-T6 cells in vitro and also evaluates the activity of ZK14 in CCl4-induced male hepatofibrotic rats in vivo.
Figure 1.
Chemical structure of DDB and ZK14.
Materials and methods
Reagents
ZK14, DDB and furoxan monomer (furoxan) were gifts of Prof Yi-hua ZHANG4. ZK14 was obtained in good yield (98%) and confirmed by 1H NMR spectra14. It was dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 100 mmol/L and stored at −20 °C. At use, it was freshly diluted with phosphate-buffered saline containing 0.1% DMSO. For each experiment, controls were treated with an identical amount of DMSO.
Cell culture
The HSC-T6 cell line was obtained from Prof SL FRIEDMAN (Liver Disease Research Center of San Francisco General Hospital, CA, USA). HSC-T6 cells were routinely cultured in DMEM (Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/mL penicillin and 100 μg/mL streptomycin. Cultures were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37 °C; 0.25% trypsin was used for cell passage. The human liver cell line L-02 (Nanjing KeyGen, China) was cultured in RPMI-1640 medium (Gibco, USA).
Viability tests and in vitro screening of compounds
HSC-T6 cells were cultured at a density of 5×104 cells/mL in 100 μL culture medium in 96-well microplates. After 6, 12, and 18 h of treatment with different doses of various compounds, the culture medium was removed and 20 μL MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide, 5 mg/mL) (MTT, Sigma, USA) was added. Four hours later, the supernatant was discarded and 100 μL DMSO was added to each well. The mixture was shaken and OD values were measured at 570 nm using a Universal Microplate Reader (EL800, BIO-TEK Instruments Inc). The extent of inhibition of HSC-T6 proliferation and the IC50 of 55 compounds were calculated using the results of the MTT assay; primary screening and evaluation were also performed.
Annexin-V/PI double-staining assay
After treatment with 50 μmol/L ZK14 for 12 h, cells were washed and resuspended in PBS. Apoptotic cells were identified using the Annexin V-FITC Apoptosis Detection kit (BD, USA) according to the manufacturer's instructions; early apoptotic cells were labeled with Annexin-V+/PI−.
Terminal deoxynucleotidyl transferase-mediated digoxigenin-11-dUTP nick end labeling (TUNEL) assay
After treatment with ZK14, HSC-T6 cells were collected and 50–100 μL of cell suspension was spread onto glass slides. Air-dried samples were fixed with freshly prepared fixation solution for 1 h at 15–25 °C; the slides were then washed with phosphate buffered saline (PBS), incubated with blocking solution for 10 min at 15–25 °C, washed with PBS, incubated in permeabilization solution for 2 min on ice (2–8 °C) and rinsed twice with PBS. The area around the samples was dried and 50 μL TUNEL reaction mixture from the TUNEL Apoptosis Detection Kit (Nanjing KeyGen, China) was added to each sample.
Assessment of mitochondrial membrane potential (MMP)
Pretreated HSC-T6 cells were collected and suspended in 1 mL of complete medium containing 10 μg/mL JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanineiodide), a fluorescent dye (purchased from Nanjing KeyGen, China), for 30 min at 37 °C. To assess the mitochondrial potential transition, 10 000 cells/sample stained by JC-1 were observed using fluorescence microscopy (Leica, Germane) and flow cytometry.
Colorimetric assay for caspase activities
HSC-T6 cells treated with ZK14 were collected and caspase activity of effectors of caspase 3 and 9 was determined using the Caspase Colorimetric Assay Kit (Nanjing KeyGen, China).
Animals and treatments
Male Sprague-Dawley rats weighing 150–180 g were obtained from the animal facility of Nanjing Military Hospital (SCXK2003-0004). All animals were housed in conventional cages under controlled conditions of temperature (23±3 °C) and relative humidity (50%±20%), with light illumination for 12 h/day. The animals were allowed access to food and tap water ad libitum throughout the acclimatization and experimental periods. All experimental protocols described in this study were in accordance with the NIH Guide for the Care and Use of Laboratory Animals, NIH publication No 85–23, 1985.
Following acclimatization for one week after arrival, forty-eight rats were randomly divided into two groups, a normal control group (n=6) and an administration group (n=42). Animals in the administration group were given drinking water containing phenobarbital sodium (350 mg/L)15 and were injected intraperitoneally with 0.025 mL CCl4 (1:6 with olive oil) three times weekly. Rats in the normal control group were injected with the same volume of vehicle. After six weeks, four rats were dead including two that did not tolerate modeling and two that were sacrificed to confirm successful modeling. The remaining thirty-eight rats with successful modeling were randomly divided into a model control group, a ZK14 high dose group (20 mg/kg), a ZK14 low dose group (10 mg/kg) and a DDB (5 mg/kg) group. All compounds were suspended with 0.5% sodium carboxymethylcellulose and intragastrically administered. To maintain etiology stimulation, CCl4 was injected once a week during drug administration. After four weeks, blood was collected from all rats by bleeding from the ophthalmic artery and vein. Serum samples were prepared by centrifugation and stored at -70 °C. Liver tissue specimens were fixed in neutral formalin and embedded in paraffin.
Serum activities of AST and ALT were assayed by standard enzymatic methods, HA and PCIII concentrations were measured with radioimmunoassay (RIA) using a commercial kit (Navy Medical Institute, Shanghai, China). Liver tissue samples were fixed in 40 g/L paraformaldehyde and embedded in paraffin. Hematoxylin and eosin (HE) staining was performed according to standard procedures by the pathology department of Southeast University. The semi-quantitative method used for scoring severity of fibrosis has been previously described16. Slides were scored independently by two pathologists with no knowledge of liver sources. The degree of fibrosis was expressed as the mean of ten different fields in each slide.
Measurement of nitrite/nitrate levels in vitro and in vivo
Because of its active chemical properties, NO was metabolized to NO2- and NO3− in the culture medium, and NO2− was transformed to NO3−. NO3− was deoxidized to NO2− by nitrate reductase in the NO assay kit (JianCheng, Nanjing, China), and the concentration of total NO2−, representing the level of NO released from ZK14, was measured by optical density (OD) assay. A solution of ZK14 (20 μL) in DMSO was added to phosphate buffer (pH 7.4). The final concentration of the compound was 1 μmol/L. After different times of incubation at 37 °C, the OD at 540 nm, which represented NO level released from ZK14 in vitro, was measured. The serum NO level in hepatic fibrosis rats, representing NO released from ZK14, was also assessed.
Statistical analysis
Quantitative data were presented as mean±SD and compared using the Student's t-test. The rank-sum test was used for histopathological fibrosis scores. P<0.05 was considered statistically significant.
Results
NO donor ZK14 inhibited HSC-T6 proliferation
HSC-T6 cells were exposed to different compounds in vitro and proliferation of the cells was assessed using the MTT method. Several compounds showed inhibitory effects on HSC-T6 cells; ZK14, which had the lowest IC50 (half-maximal inhibitory concentration) (17.83 μmol/L; data not shown) was selected as the target compound.
NO donor ZK14 induced HSC-T6 apoptosis
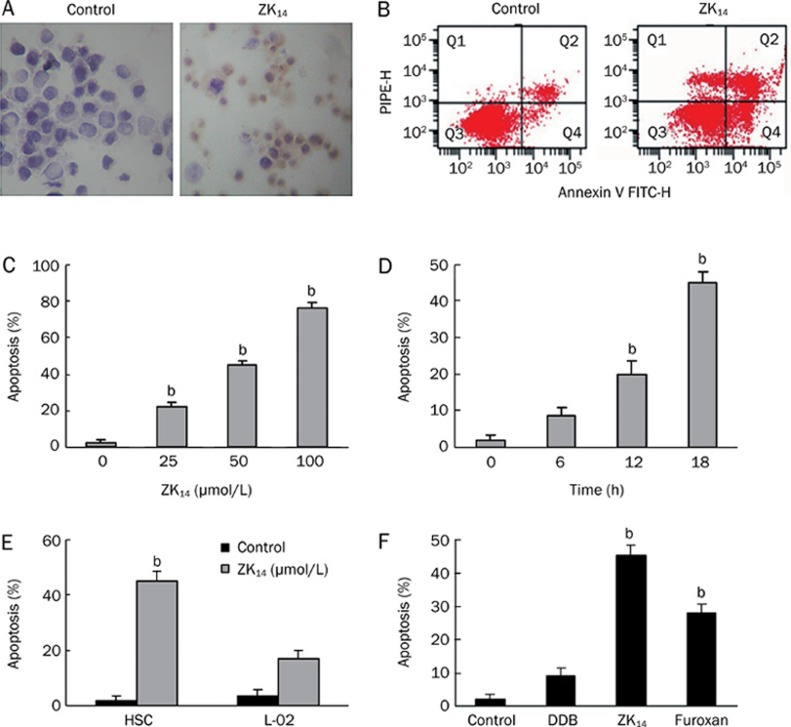
HSC-T6 cells were exposed to NO donor ZK14 at different concentrations and for different amounts of time and apoptosis was quantitated via TUNEL and Annexin-V/PI double-staining assays (Figure 2A, 2B). The apoptosis was both concentration- and time-dependent, with up to 70% of the cells becoming apoptotic when exposed to 100 μmol/L ZK14 for 18 h (Figure 2C). In all subsequent experiments, the dose of ZK14 (50 μmol/L) consistently resulted in an apoptosis rate of about 40% on 18 h exposure (Figure 2C, 2D). ZK14 of 1 μmol/L generated a concentration of 0.06 μmol/L of nitrite in 350 min, as measured by nitric reduction assay.
Figure 2.
Effect of ZK14 on HSC-T6 cells in vitro. (A) TUNEL assay of DNA integrity. HSC-T6 cells were pretreated with 50 μmol/L ZK14 for 12 h. 3′-OH DNA ends were stained dark brown with DAB. (B) Flow cytometry of apoptosis. HSC-T6 cells displayed increased Annexin-V positivity after exposure to ZK14 50 μmol/L for 12 h. (C) Concentration-dependent apoptosis. HSC-T6 cells were exposed to increasing concentrations of ZK14 (0–100 μmol/L) for 18 h. Apoptosis was quantitated by flow cytometry. (D) Time-dependent apoptosis. HSC-T6 cells were exposed to ZK14 50 μmol/L for increasing times. Apoptosis was quantitated by flow cytometry. (E) Apoptosis of HSC-T6 cells and hepatocytes L-02 exposed to ZK14 50 μmol/L for 18 h. (F) HSC-T6 cells were exposed to 50 μmol/L ZK14, furoxan, and DDB. n=3. Mean±SD. bP<0.05 vs control.
Since NO is a highly reactive free radical, we sought to test whether induction of apoptosis by an NO donor could result in injury to normal liver tissue cells. When exposed to 50 μmol/L ZK14, the apoptotic rate of L-02 cells was relatively low (17%) compared with that of HSC-T6 (Figure 2E). Exposure to ZK14 resulted in a higher rate of apoptosis (45%) than exposure to either furoxan (28%) or DDB (9%); these experiments also revealed a synergistic effect of furoxan and DDB on apoptosis induction in HSC-T6 cells (Figure 2F).
NO donor ZK14-mediated apoptosis is related to mitochondrial dysfunction and caspase activation
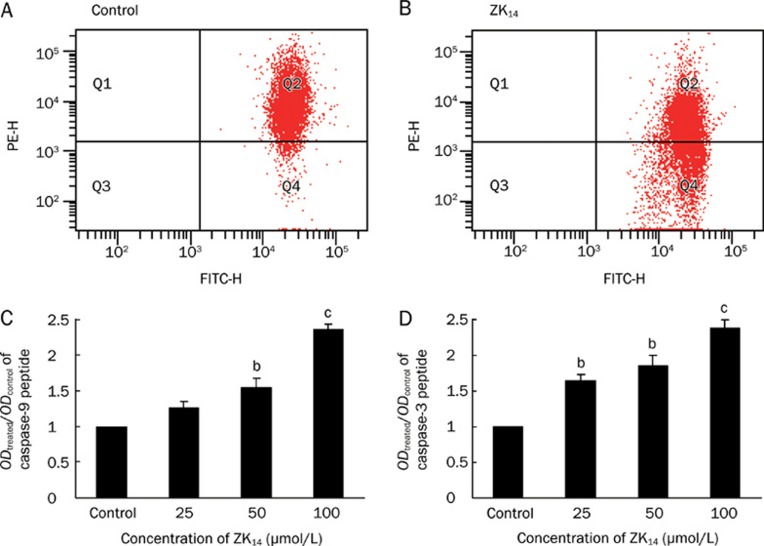
It has been shown that integrity of mitochondria plays an important role in programmed cell death and that disruption of MMP enhances apoptosis17. To probe the mechanism by which NO donor ZK14 induced HSC-T6 apoptosis, HSC-T6 cells were treated with ZK14 (50 μmol/L) for 12 h and stained with JC-1, a potential-sensitive fluorescent dye. When apoptosis was induced in cells, the mitochondrial membrane was depolarized and JC-1 was released from mitochondria to cytoplasts that gave off weak red and green fluorescence. In normal cells, JC-1 bound to the inner membrane of mitochondria and gave off bright red fluorescence as polymers. FCM analysis showed that the percentage of normal cells labeled by JC-1 was 96.2% (Figure 3A). However, in apoptotic cells, due to depolarization of MMP, JC-1 was released from mitochondria to cytoplasts and decomposed to monomers; as a result, the percentage of cells labeled by JC-1 decreased to 59.7% (Figure 3B). The decreased number of cells labeled by JC-1 in the ZK14-treated group indicated loss of MMP.
Figure 3.
(A, B) Normal HSC-T6 cell percentage labeled with JC-1 in the inner membrane of mitochondria was 96.2%, and decreased to 59.7% when treated with 50 μmol/L ZK14 for 18 h. The decreased cells labeled with JC-1 revealed the loss of MMP and subsequently with the apoptosis of HSC-T6 cells. (C, D) Caspase 9 and 3 activity was measured and represented by ratios of ODtreated/ODcontrol after exposure to ZK14 for 18 h. After treated with 25, 50, and 100 μmol/L of ZK14 for 12 h, caspase 9 activity increased to 1.26, 1.55, and 2.36, and caspase-3 activity increased to 1.64, 1.85 and 2.38. n=3. Mean±SD. bP<0.05, cP<0.01 vs control.
Caspases (cysteinyl aspartate proteinases) play a major role in apoptosis. Caspase 9 is an upstream molecule, while caspase 3 is the key executive molecule in the cascade reaction, coupling caspase-specific peptides to chromophoric groups. After treatment with ZK14 (0, 25, 50, or 100 μmol/L) for 12 h, the activity of caspase 9 increased from 1 to 1.26, 1.55, and 2.36, respectively (Figure 3C), and the activity of caspase 3 increased to 1.64, 1.85, and 2.38 (Figure 3D). This result shows that the apoptosis induced in HSC-T6 cells was related to enhanced activity of caspases 3 and 9.
NO donor ZK14 ameliorated liver injury and fibrosis in CCl4-induced rats
Table 1 shows levels of liver enzymes and fibrosis markers in CCl4-treated male rats. Serum levels of ALT and AST are sensitive markers of hepatocyte function. Serum levels of HA and PCIII are surrogate markers of liver fibrogenesis. Compared with normal rats, the model control rats had obvious liver inflammation and formed hepatic pseudolobules due to collagen deposition; these animals also exhibited higher levels of serum ALT, AST, HA, and PCIII (Table 1 and Figure 4). These results show that hepatic fibrosis was successfully induced in rats. ZK14 (20 mg/kg) and DDB (5 mg/kg) significantly decreased elevated serum ALT and AST levels, while ZK14 (10 mg/kg) had no obvious effect. ZK14 (20 mg/kg) caused a significant decrease in serum levels of HA and PCIII, while DDB (5 mg/kg) and ZK14 (10 mg/kg) did not have significant effects (Table 1).
Table 1. Effects of ZK14 on serum ALT, AST, HA, PCIII, and NO level in hepatic fibrosis rats induced by CCl4. Mean±SD. bP<0.05, cP<0.01 vs normal. eP<0.05, fP<0.01 vs model.
| Groups | Dose (mg/kg) | n | ALT (U/L) | AST (U/L) | HA (ng/mL) | PCIII (ng/mL) | NO (μg/L) |
|---|---|---|---|---|---|---|---|
| Normal | / | 6 | 22.73±3.5 | 113.35±11.35 | 248.19±37.53 | 7.85±2.15 | 50.69±15.37 |
| Model | / | 8 | 50.12±12.56b | 222.83±25.36b | 1017.45±52.84c | 35.36±9.70c | 42.34±14.21 |
| ZK14 | 20 | 10 | 35.74±7.05e | 145.19±34.77e | 422.27±56.23f | 20.93±6.73f | 154.84±29.49c |
| ZK14 | 10 | 10 | 47.16±25.72 | 208.41±32.45 | 957.62±52.01 | 39.88±8.45 | 96.67±20.45b |
| DDB | 5 | 10 | 27.47±30.37e | 136.79±30.43e | 967.46±66.32 | 32.72±8.78 | 46.88±12.20 |
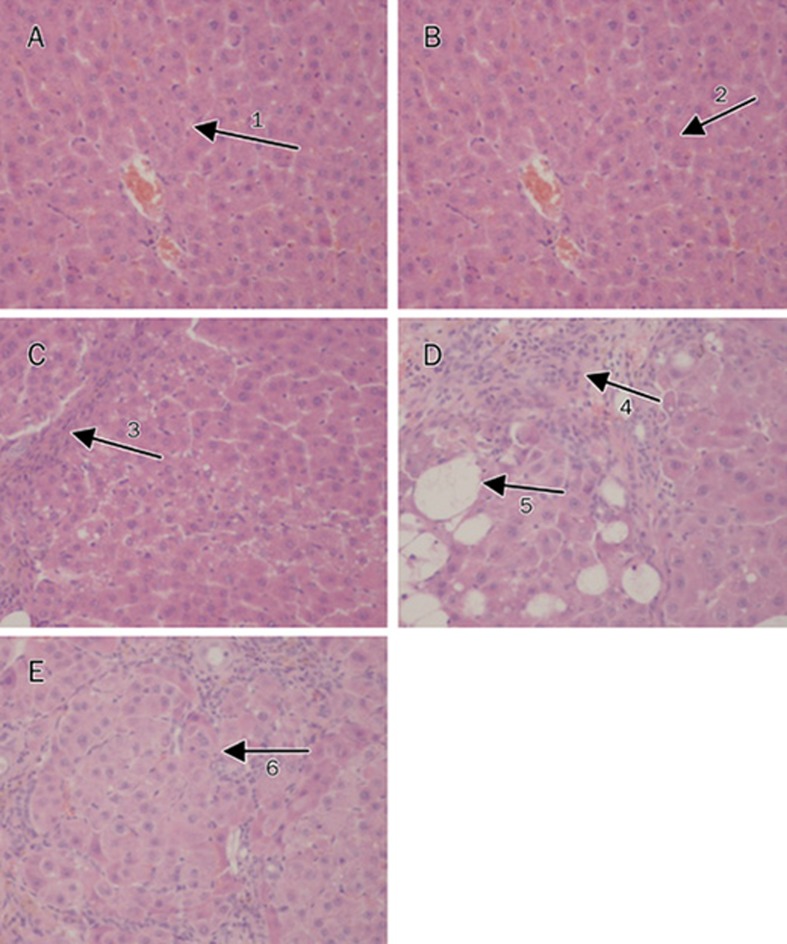
Figure 4.
The photomicrographs of liver section taken from rats. (A) Normal group, saline injection only, normal hepatic lobule (arrow 1). (B) Model group, CCl4 (0.025 mL) injection only, obvious hepatic pseudolobule (arrow 2). (C) ZK14 high dose group, CCl4+ZK14 (20 mg/kg), centrilobular fibrosis (arrow 3). (D) ZK14 low dose group, CCl4+ZK14 (10 mg/kg), centrilobular fibrosis (arrow 4) and fatty degeneration (arrow 5). (E) DDB group, CCl4+DDB (5 mg/kg), hepatic pseudolobule (arrow 6). HE stain, ×200.
On hepatic histological examination, it could be seen that high dose of ZK14 (20 mg/kg) significantly inhibited the production of collagen fibers in the livers of rats (Table 2). As shown in Figure 4, the structure of liver tissues was normal in the normal group (Figure 4A). In tissues of the hepatic fibrosis model group, shown in Figure 4B, fibrous septa encircled the hepatic lobule, hepatic pseudolobules were formed, and severe centrilobular fibrosis and fatty changes were also observed. There was mild collagen proliferation. In animals of the model group that received high dose of ZK14, the structure of the hepatic lobules was renewed (Figure 4C). There was no obvious amelioration either in the low dose ZK14 group or in the DDB group (Figure 4D, 4E); in these animals, significant fatty change and pseudolobule were observed.
Table 2. Effects of ZK14 on the pathologic grading of hepatic fibrosis rats induced by CCl4. P<0.01, compared with normal group. P<0.05, compared with model group.
| Groups | Dose (mg/kg) | n | Pathologic grading of hepatic fibrosis |
P | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | ||||
| Normal | / | 6 | 10 | 0 | 0 | 0 | 0 | – |
| Model | / | 8 | 0 | 0 | 3 | 4 | 3 | <0.01 |
| ZK14 | 20 | 10 | 0 | 1 | 6 | 3 | 0 | <0.05 |
| ZK14 | 10 | 10 | 0 | 1 | 2 | 5 | 2 | – |
| DDB | 5 | 10 | 0 | 3 | 6 | 2 | – | |
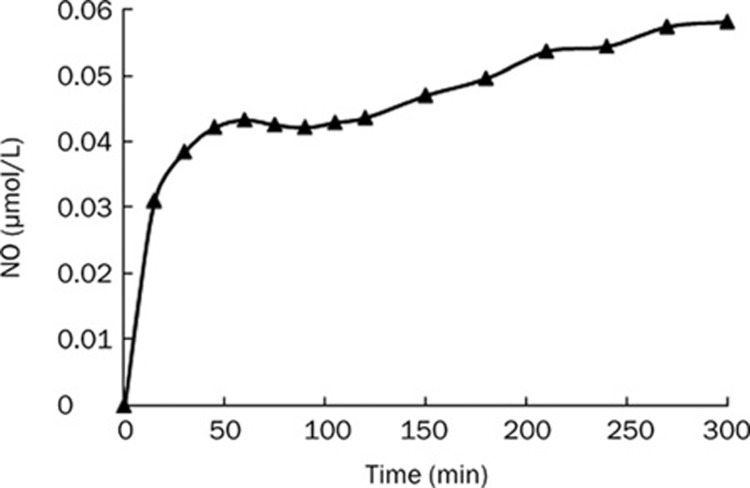
ZK14 released NO effectively in vitro and in vivo
As Figure 5 shows, NO could be stably released from ZK14 in vitro. The content of NO reached a maximum value of about 0.06 μmol/L and maintained stable levels over the subsequent 350 min. As shown in Table 1, serum NO level increased significantly in ZK14 (20 mg/kg) and ZK14 (10 mg/kg) groups compared with the normal group, while NO levels in the model and DDB groups did not change.
Figure 5.
NO releasing from ZK14 in vitro. ZK14 was dissolved in DMSO and reached the concentration of 1 μmol/L. The maximum releasing content of NO was about 0.06 μmol/L, and maintained stable levels in subsequent 350 min.
Discussion
The pharmacological effects of compound ZK14, a novel nitric oxide-donating DDB derivative, were examined in vitro and in vivo. Our studies showed significant apoptosis-inducing activity of ZK14 in HSC-T6 in vitro, as reflected by decreased MMP and increased caspase-3 and 9 activities. Furthermore, high doses of ZK14 (20 mg/kg) showed anti-fibrotic as well as hepatocyte-protective activity in CCl4-induced male hepatofibrotic rats.
NO is a highly reactive free radical capable of mediating a multitude of reactions; it is produced by the catalytic reaction of L-lysine via nitric oxide synthases or generated from synthetic NO-releasing compounds such as furoxan, nitrate, diazeniumdiolate and others. Compound ZK14, which we synthesized with the NO donor furoxan, could release NO stably in vitro in 350 min. Furthermore, serum NO level increased remarkably in animals treated with high or low doses of ZK14. These results show that NO can be released from ZK14 both in vitro and in vivo, and demonstrate that ZK14 is an efficacious NO donor.
NO generated from endothelial NO synthase (eNOS) in turn exerts paracrine effects on adjacent HSCs, culminating in the inhibition of vasoconstriction, proliferation, and migration18, 19, 20. Recent studies suggest that NO promotes HSC apoptosis through a signaling mechanism that involves mitochondria and that occurs independently of caspase activation21.
Our study in vitro showed that the apoptosis induced by NO donor ZK14 was both concentration- and time-dependent. When exposed to ZK14 (50 μmol/L), the apoptotic rate in HSC-T6 cells was about 40%, while that in L-02 cells was moderately low (17%). This shows that apoptosis induced by ZK14 is not cell-specific, and that to improve cell-selectivity, synthesis of a different compound is needed.
NO can pass through cell membranes due to its water-soluble and lipid-soluble properties; inside cells, it acts on target molecules and induces apoptosis22, 23, 24 in various cells and tissues. NO can directly induce cytochrome c release through MMP loss, and released cytochrome c can activate the caspase-dependent apoptotic signaling cascade25. NO-induced apoptosis occurs by two separate pathways: caspase-dependent and caspase-independent. The caspase-dependent pathway can be either intrinsic or extrinsic in nature. The intrinsic pathway is stimulated by apoptotic stimuli, including radiation and the 2–5A/RNase L/RNase L inhibitor (RNI), leading to the loss of MMP and release of cytochrome c into the cytosol. Along with ATP, apoptotic protease activating factor-1 (Apaf-1) and procaspase-9, cytochrome c directs the activation of the initiator caspase 9 followed by the processing of procaspase-3 and its activation. Thus, apoptosis occurs downstream of active caspase 3.
When treated with ZK14, the MMP of HSC-T6 cells decreased and the activities of caspase 3 and 9 increased. This result shows that the mechanism of apoptosis induced by ZK14 in HSC-T6 is related to the intrinsic caspase-dependent pathway. It is likely that when NO was released from ZK14, MMP decreased and cytochrome c activated caspase 9 and subsequently caspase-3, which initiated apoptosis in HSC-T6 cells.
Serum levels of HA and PCIII are surrogate markers of liver fibrogenesis. Significant amelioration in serum markers of liver fibrogenesis and in hepatic histological pathology occurred in the ZK14 high-dose group (20 mg/kg), while no significant effects were observed in the DDB group (5 mg/kg) or the ZK14 low-dose group (10 mg/kg). This result suggests that apoptosis induced by NO generated from ZK14 resulted in subsequent elimination of activated HSCs and extracellular matrix, with collagen fibers being destroyed and absorbed and the structure of the hepatic lobules renewed.
Serum levels of ALT and AST are sensitive markers of hepatocyte function. We observed significant decreases in serum ALT and AST levels in the ZK14 high dose group (20 mg/kg) and in the DDB group (5 mg/kg), with no obvious effect in the ZK14 low dose group (10 mg/kg). This suggests a protective effect of ZK14 on liver function in hepatic fibrosis due to mother nucleus DDB.
As a synthetic commercial hepatoprotectant, DDB has been widely used to treat chronic viral hepatitis B patients in China for more than 20 years. Oral administration of DDB significantly prevented the occurrence of erythromycin-induced liver damage26. DDB directly inhibited IFN-alpha signaling-mediated replication of HBV in infected hepatocytes27. DDB could exert its biochemical effects through enhancement of antioxidant enzyme activities and reduction of glutathione levels as well as by decreasing lipid peroxides28, 29. DDB might inhibit inflammatory responses in association with reduction of NF-kappa B activation through prevention of I-kappa B alpha degradation and subsequent TNF-alpha production30.
By contrast, apoptosis induced by ZK14 in HSC-T6 cells in vitro was attributed to NO; DDB treatment did not induce apoptosis effectively (Figure 2F). We considered that by virtue of its anti-inflammatory and anti-immune properties, DDB would exert its role of protecting liver and reducing enzyme levels in vivo rather in vitro. In animal experiments, high-dose of ZK14 and DDB exhibited preferable liver protection, while ZK14 did not exert its protecting effects against apoptosis of L-02 cells in vitro.
Depending on its concentration, NO can have pro- or anti-apoptotic properties. High NO concentrations promote apoptosis in most cases, whereas low NO concentrations can result in resistance to apoptosis24. There was no significant reduction in liver fibrosis in the ZK14 low-dose group (10 mg/kg). This might be because a low NO concentration released in this group was insufficient to induce apoptosis, and instead inversely induced proliferation of HSC and caused aggravation of liver fibrosis.
In conclusion, we performed a primary screening and evaluation of ZK14 as a novel NO donor compound and found it was effective both in vitro and in vivo. ZK14 promoted apoptosis in HSC-T6 cells by releasing NO and activating a signaling mechanism that involved mitochondrial dysfunction and caspase activation. Furthermore, ZK14 could alleviate the syndrome of hepatic fibrosis in vivo, and protect liver function by DDB. These results demonstrate that ZK14, a novel nitric oxide-donating DDB derivative, has effective antifibrotic activity both in vitro and in vivo. We hope our experiments will provide novel support for the use of apoptosis induction for active HSC in therapy of hepatic fibrosis.
Author contribution
Hui JI and Li DAI designed research; Li DAI performed research, analyzed data and wrote the paper; Yi-hua ZHANG and Xiang-wen KONG contributed key compounds.
Acknowledgments
This work was supported in part by the Research Fund for the Doctoral Program of Higher Education (No 20070316007).
References
- Yao HW, Li J, Chen JQ, Xu SY. Inhibitory effect of leflunomide on hepatic fibrosis induced by CCl4 in rats. Acta Pharmacol Sin. 2004;25:915–20. [PubMed] [Google Scholar]
- Friedman SL. Liver fibrosis – from bench to bedside. J Hepatol. 2003;38:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, et al. Mechanisms of spontaneous resolution of rat liver fibrosis: hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–49. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MDA, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic fibrosis-overview. Toxicology. 2008;3:120–9. doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Dekel R, Zvibel I, Brill S, Brazovsky E, Halpern Z, Oren R. Gliotoxin ameliorates development of fibrosis and cirrhosis in a thioacetamide rat model. Dig Dis Sci. 2003;48:1642–7. doi: 10.1023/a:1024792529601. [DOI] [PubMed] [Google Scholar]
- Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, et al. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–98. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- Beljaars L, Meijer DK, Poelstra K. Targeting hepatic stellate cells for cell-specific treatment of liver fibrosis. Front Biosci. 2002;7:e214–22. doi: 10.2741/A917. [DOI] [PubMed] [Google Scholar]
- Hagens WI, Olinga P, Meijer DK, Groothuis GM, Beljaars L, Poelstra K. Gliotoxin non-selectively induces apoptosis in fibrotic and normal livers. Liver Int. 2006;26:232–9. doi: 10.1111/j.1478-3231.2005.01212.x. [DOI] [PubMed] [Google Scholar]
- Fu TB, Liu GT. A research on protective effect of biphenyl dimethyl dicaboxylate on injured hepatocytes. Nat Med J China. 1990;7:201–4. [PubMed] [Google Scholar]
- Wang MR, Jia KM. A study on protective effect of biphenyl dimethyl dicaboxylate (BOD) on ultrastructure of human fetal hepatocytes injured by CCl4. Med J Chin People's Liberation Army. 1990;15:436–8. [Google Scholar]
- Abdel-Hameid NA. Protective role of dimethyl diphenyl bicarboxylate (DDB) against erythromycin induced hepatotoxicity in male rats. Toxicol In Vitro. 2007;21:618–25. doi: 10.1016/j.tiv.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Abdel-Salam OM, Sleem AA, Morsy FA. Effects of biphenyldimethyl-dicarboxylate administration alone or combined with silymarin in the CCl4 model of liver fibrosis in rats. ScientificWorldJournal. 2007;7:1242–55. doi: 10.1100/tsw.2007.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XW, Zhang YH, Dai L, Ji H. Synthesis and biological evaluation of nitric oxide-releasing sixalkoxyl biphenyl derivatives as anticancer agents. Chin Chem Lett. 2008;19:149–52. [Google Scholar]
- McLean EK, McLean AE, Sutton PM. Instant cirrhosis. An improved method for producing cirrhosis of the liver in rats by simultaneous administration of carbon tetrachloride and phenobarbitone. Br J Exp Pathol. 1969;50:502–6. [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wei W, Wang NP, Wu CY, Yan SX, Yue L, et al. Effects of total glucosides of peony on immunological hepatic fibrosis in rats. World J Gastroenterol. 2005;11:2124–9. doi: 10.3748/wjg.v11.i14.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao AM, Ikejima T, Tashiro S, Onodera S, Zhang WG, Wu YL. Involvement of mitochondria and caspase pathways in N-demethyl-clarithromycin-induced apoptosis in human cervical cancer HeLa cell. Acta Pharmacol Sin. 2006;27:1622–9. doi: 10.1111/j.1745-7254.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- Perri RE, Langer DA, Chatterjee S, Gibbons SJ, Gadgil J, Cao S, et al. Defects in cGMP-PKG pathway contribute to impaired NO-dependent responses in hepatic stellate cells upon activation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G535–G542. doi: 10.1152/ajpgi.00297.2005. [DOI] [PubMed] [Google Scholar]
- Failli P, De FR, Caligiuri A, Gentilini A, Romanelli RG, Marra F, et al. Nitrovasodilators inhibit platelet-derived growth factor-induced proliferation and migration of activated human hepatic stellate cells. Gastroenterology. 2000;119:479–92. doi: 10.1053/gast.2000.9354. [DOI] [PubMed] [Google Scholar]
- Lee JS, Decker N, Chatterjee S, Yao J, Friedman S, Shah V. Mechanisms of nitric oxide interplay with Rho GTPase family members in modulation of actin membrane dynamics in pericytes and fibroblasts. Am J Pathol. 2005;166:1861–70. doi: 10.1016/S0002-9440(10)62495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer DA, Das A, Semela D, Kang-Decker N, Hendrickson H, Bronk SF. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology. 2008;47:1983–93. doi: 10.1002/hep.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Tian H, Tang CS, Jin HF, Du JB. Nitric oxide modulates hypoxic pulmonary smooth muscle cell proliferation and apoptosis by regulating carbon monoxide pathway. Acta Pharmacol Sin. 2007;28:28–35. doi: 10.1111/j.1745-7254.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- Vieira H, Kroemer G. Mitochondria as targets of apoptosis regulation by nitric oxide. IUBMB Life. 2003;55:613–6. doi: 10.1080/15216540310001639652. [DOI] [PubMed] [Google Scholar]
- Rishi L, Dhiman R, Raje M, Majumdar S. Nitric oxide induces apoptosis in cutaneous T cell lymphoma (HuT-78) by downregulating constitutive NF-κB. Biochim Biophys Acta. 2007;1770:1230–9. doi: 10.1016/j.bbagen.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Bonavida B, Khineche S, Huerta-Yepez S, Garbán H. Therapeutic potential of nitric oxide in cancer. Drug Resist Updat. 2006;9:157–73. doi: 10.1016/j.drup.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Abdel-Hameid NA. Protective role of dimethyl diphenyl bicarboxylate (DDB) against erythromycin induced hepatotoxicity in male rats. Ai Zheng. 2006;25:1464–9. doi: 10.1016/j.tiv.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Joo SS, Won TJ, Kim MJ, Hwang KW, Lee do I.Interferon signal transduction of biphenyl dimethyl dicarboxylate/amantadine and anti-HBV activity in HepG2 2.2.15 J Biochem Mol Biol 200538300–6.15943905 [Google Scholar]
- El-Beshbishy HA. The effect of dimethyl dimethoxy biphenyl dicarboxylate (DDB) against tamoxifen-induced liver injury in rats: DDB use is curative or protective. East Mediterr Health J. 2002;8:95–104. doi: 10.5483/bmbrep.2005.38.3.300. [DOI] [PubMed] [Google Scholar]
- el-Sawy SA, el-Shafey AM, el-Bahrawy HA. Effect of dimethyl diphenyl bicarboxylate on normal and chemically-injured liver. Yao Xue Xue Bao. 2001;36:493–7. [PubMed] [Google Scholar]
- Kim SG, Kim HJ, Choi SH, Ryu JY. Inhibition of lipopolysaccharide-induced I-kappaB degradation and tumor necrosis factor-alpha expression by dimethyl-4,4′-dimethoxy-5,6,5′,6′-dimethylene dioxybiphenyl-2,2′-dicarboxylate (DDB): minor role in hepatic detoxifying enzyme expression. Mol Cell Biochem. 2000;205:111–4. doi: 10.1034/j.1600-0676.2000.020004319.x. [DOI] [PubMed] [Google Scholar]