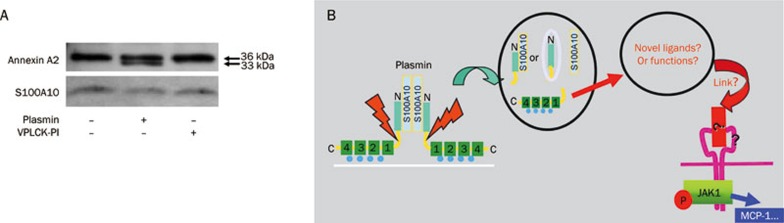
Figure 2.
Proteolytic activity of plasmin is required for the cleavage of annexin A2 in monocytes. (A) Monocytes were stimulated for 30 min with 0.43 CTA U/mL plasmin or the equivalent amount of catalytically inactivated plasmin (VPLCK-PL). After treatment, cells were lysed, and proteins were separated and visualized by immunoblotting with antibodies against annexin A2 and S100A10. The results shown are representative of at least three independent experiments. (B) Hypothesis of the possible function of the proteolytic cleavage and dissociation of the plasmin receptor. According to consensus models, the annexin A2 heterotetramer interacts with the extracellular surface of the membrane; however, AA2t is not inserted into the membrane. After proteolytic cleavage by plasmin and dissociation of the receptor, this receptor may generate four new proteolytic fragments: either A2NPd and A2Ct, or A2Nt, A2Ct, and S100A10 alone. These fragments may interact with other proteins to form new complexes, or they may serve as novel ligands for transmembrane receptors. This result may initiate the known downstream signaling via JAKs/STATs, inducing the release of the inflammatory chemokine MCP-1.