Abstract
Aim:
To examine the role of protein L-isoaspartyl O-methyltransferase (PIMT; EC 2.1.1.77) on the secretion of Aβ peptides.
Methods:
HEK293 APPsw cells were treated with PIMT siRNA or adenosine dialdehyde (AdOX), a broad-spectrum methyltransferase inhibitor. Under the conditions, the level of Aβ secretion and regulatory mechanism by PIMT were examined.
Results:
Knock-down of PIMT and treatment with AdOX significantly increased Aβ40 secretion. Reductions in levels of PIMT decreased the secretion of soluble amyloid precursor protein alpha (sAPPα) without altering the total expression of APP or its membrane-bound C83 fragment. However, the levels of the C99 fragment generated by β-secretase were enhanced. Moreover, the decreased secretion of sAPPα resulting from PIMT knock-down seemed to be linked with the suppression of the expression of α-secretase gene products, α-disintegrin and metalloprotease 10 (ADAM10) and ADAM17, as indicated by Western blot analysis. In contrast, ADAM10 was not down-regulated in response to treatment with the protein arginine methyltransferase (PRMT) inhibitor, AMI-1.
Conclusion:
This study demonstrates a novel role for PIMT, but not PRMT, as a negative regulator of Aβ peptide formation and a potential protective factor in the pathogenesis of AD.
Keywords: Alzheimer's disease, β-amyloid protein, L-isoaspartyl O-methyltransferase, soluble amyloid precursor protein alpha, ADAM10, ADAM17
Introduction
Alzheimer's disease (AD), the most common neurodegenerative disease, is characterized by progressive memory loss and other cognitive impairments1, 2. Neuropathological hallmarks of AD include the deposition of amyloid beta (Aβ) peptides, which are organized in senile plaques. In addition, AD is characterized by the accumulation of phosphorylated tau proteins, which are arranged in neurofibrillary tangles (NFTs)2. Aβ peptides are generated through the proteolysis of the amyloid precursor protein (APP). In the amyloidogenic pathway, β-secretase cleaves APP to produce soluble amyloid precursor protein beta sAPPβ and a C99 fragment. Membrane-bound C99 can be further processed by γ-secretase to produce Aβ peptides3, 4. As an alternative, non-amyloidogenic pathway, α-secretase can cleave within the Aβ region to produce a sAPPα fragment and a C83 fragment4.
High levels of homocysteine (HCY) can lead to increased concentrations of S-adenosylhomocysteine (SAH), a strong methyltransferase inhibitor5. This up-regulation of SAH results in an overall decrease in the activity of S-adenosylmethionine (SAM)-dependent methyltransferases. Increased SAH levels in the brain tissue of patients with AD has been associated with the inhibition of catechol-O-methyltransferase (COMT) and phenylethanolamine-N-methyltransferase (PNMT), two enzymes that are widely distributed throughout the human brain6. Treatment of Neuro-2a neuroblastoma cells with SAH has been shown to inhibit protein phosphatase 2A methyltransferase (PPMT), resulting in decreased methylation of protein phosphatase 2A7. SAH treatment has also been associated with the increased accumulation of APP and phosphorylated tau and with increased Aβ secretion8. Protein L-isoaspartyl methylation is also essential for the maintenance of neural activity in the central nervous system (CNS). Deficiency of protein L-isoaspartyl O-methyltransferase (PIMT, EC2.1.1.77), an enzyme that catalyzes the transfer of an active methyl group from SAM to L-isoaspartate and D-isoaspartate, leads to fatal progressive epileptic disease9. Alterations in the SAM/SAH ratio, which is relevant to the overall excitatory state of neurons, have been reported in PIMT-deficient mice10. Previous studies have identified deposits of Aβ peptides with isoaspartates in brain tissue isolated from AD patients and PIMT knock-out mice, suggesting a potential pathophysiological role in progressive neurodegeneration10, 11. In patients with AD, PIMT is up-regulated in degenerating neurons and is localized in NFTs10. Despite the increasing evidence supporting a role for PIMT in neurodegeneration, the mechanism by which PIMT modulates Aβ peptide generation in AD pathogenesis remains unclear. To uncover the mechanism whereby PIMT exerts its effects, we examined the ability of PIMT to regulate Aβ secretion in vitro.
Materials and methods
Antibodies and reagents
Adenosine dialdehyde (AdOX), SAM, and mouse anti-β-actin antibodies were purchased from Sigma-Aldrich Chemicals (St Louis, MO, USA). AMI-1 was obtained from Calbiochem (La Jolla, CA, USA). Dulbecco's modified Eagle's medium (DMEM), Opti-MEM, Dulbecco's phosphate buffered saline (DPBS), penicillin, streptomycin, and fetal bovine serum (FBS) were purchased from Gibco (Carlsbad, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Calbiochem (La Jolla, CA, USA). Moloney Murine Leukemia virus (M-MLV) reverse transcriptase and polymerase chain reaction (PCR) premix was purchased from Rexgene Biotech Co, Ltd (Ochang, Korea). All of the primers used for PCR were purchased from Bioneer (Daejeon, Korea). A mixture of Stealth™ /siRNA duplex oligoribonucleotides against PIMT and Lipofectamine™ RNAiMAX were purchased from Invitrogen (Carlsbad, CA, USA). Monoclonal mouse anti-APP (6E10) antibody was obtained from Signet Laboratories (Dedham, MA, USA). Polyclonal rabbit antibodies to ADAM9, ADAM10, and ADAM17 were obtained from Chemicon International (Temecula, CA, USA). Monoclonal mouse BACE1, monoclonal anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies, and anti-rabbit HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit anti-PIMT antisera was produced against recombinant porcine PIMT proteins as described12. The Genbank nucleotide sequence database accession number of the nucleotide sequence of the clone is AF239700.
Cell culture, drug treatment, and protein preparation
HEK293 APPsw and SH-SY5Y cells were plated on 100-mm culture dishes (Corning Incorporated, Corning, NY, USA). The dishes were filled with DMEM containing 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. The cultures were maintained at 37 °C with 5% CO2 under humidified conditions. Cells were treated with drugs (SAM, AdOX, and AMI-1) for indicated times. Vivaspin20 centrifugal filter devices (Satorius, Goettingen, Germany) were used to concentrate conditioned media (CM) from HEK293 APPsw and SH-SY5Y cells collected after drug or siRNA treatments. Cells were washed with DPBS and lysed in Pro-Prep™ protein extraction buffer for 20 min on ice. The protein concentration of each sample was quantified using a Bradford assay (Bio-Rad, Hercules, CA, USA).
Cell viability assay
To determine cell viability, cells were plated on 96-well plates at a density of 2×104 cells per well. The original media was then replaced with media containing MTT at a final concentration of 0.5 mg/mL13. Four hours later, the medium was discarded, and DMSO was added for the colorimetric assay. Absorption values were determined using an Emax microplate reader from Molecular Devices (Union City, CA, USA) with a 540-nm filter.
Reverse transcriptase-polymerase chain reaction
RNA was isolated from HEK293 APPsw cells treated with or without PIMT siRNA using TRIzol reagent (Gibco BRL) according to the manufacturer's instructions. For each RT-PCR reaction, 1 μg of RNA was used. Each sample was pre-heated to 60 °C with oligo (dT)18 primers for 10 min. One unit per milliliter of M-MLV reverse transcriptase was added. The reaction was then performed at 37 °C for 60 min with the following primers: PIMT, forward 5′-TCAGGAAGGACGATCCAACA-3′, reverse 5′-TCCTCCGGGCTTTAACTGAT-3′ and GAPDH, forward 5′-AAGGGTCATCATCTCTGCCC-3′, reverse 5′-GTGATGGCATGGACTGTGGT-3′. Amplification was carried out for 20 to 30 cycles with the following parameters: 94 °C for 30 s, 55–57 °C for 40 s, and 72 °C for 30 s. These steps were followed by a final 5 min extension step at 72 °C.
siRNA transfection
To conduct the PIMT siRNA transfection, 500 000 cells were seeded onto 100-mm culture plates. Cells were cultured for 48 h at 37 °C in culture medium containing serum, which allowed the cells to be approximately 80% confluent. Immediately prior to transfection, lipofectamine RNAiMAX was incubated with the siRNA of interest in OPTI-MEM (Gibco) at room temperature for 10 min. The cells were then incubated in this mixture for 48 h at 37 °C in fresh medium containing serum.
Immunoblotting
Twenty micrograms of protein mixed with 5×loading buffer [0.313 mol/L Tris-HCl (pH 6.8), 10% SDS, 0.05% bromophenol blue, 50% glycerol], and 20×reducing agent (2 mol/L DTT: Fermentas, Hanover, MD, USA) were boiled for 5 min and loaded onto a 10% SDS-polyacrylamide gel. After electrophoresis, proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk in 20 mmol/L Tris-HCl (pH 7.4) containing 150 mmol/L NaCl and 0.1% Tween 20 (TBS-T). They were then incubated overnight at 4 °C with primary antibodies (1:2000 for 6E10, 1:1000 for 22C11, 1:3000 for β-actin, 1:2000 for ADAM10, 1:1000 for BACE1, 1:3000 for PIMT) in non-fat milk. The membranes were washed for 10 min in TBS-T and then incubated for 2 h in non-fat milk at room temperature with horseradish peroxidase-conjugated anti-mouse/rabbit secondary antibodies. Bound antibodies were visualized with an enhanced chemiluminescence detection kit (Amersham Bioscience, Pittsburgh, PA, USA).
Cell surface biotinylation
HEK 293 APPsw cells were surface biotinylated by incubation with 2 mg/mL Sulfo-NHS-SS-Biotin (Pierce, Rockford, IL, USA) in ice-cold PBS. After 30 min, the cells were washed and quenched with PBS containing 100 mmol/L glycine. Cells were lysed in 1% NP-40 buffer and incubated with Neutravidin™ immobilized onto 6% cross-linked beaded agarose (Pierce). The beads were washed in NP-40 buffer, boiled in sample buffer, separated using SDS-PAGE, and immunoblotted with the indicated antibodies.
Aβ40 ELISA assay
A variety of Aβ peptides, ranging from 38 to 43 amino acids in length, have been shown to be secreted in response to γ-secretase activation14, 15. Aβ42 is the peptide most widely implicated in AD pathogenesis16; however, the antibody for Aβ40 was selected based on its reproducibility and accuracy in the Aβ40 ELISA kit. The CM was cleared of debris, and the secreted Aβ40 was measured using a sandwich ELISA kit (Signet Laboratory, Dedham, MA, USA) according to the manufacturer's instructions.
Statistical analysis
Quantitative analysis of Western blotting was performed by calculating the relative density of immunoreactive bands. The data are expressed as a percentage of the control values. Data are presented as the mean±SD. Each procedure was performed in three to five independent experiments. A Student's t-test analysis was used to evaluate statistical significance.
Results
PIMT siRNA and AdOX induce Aβ secretion in HEK293 APPsw cells
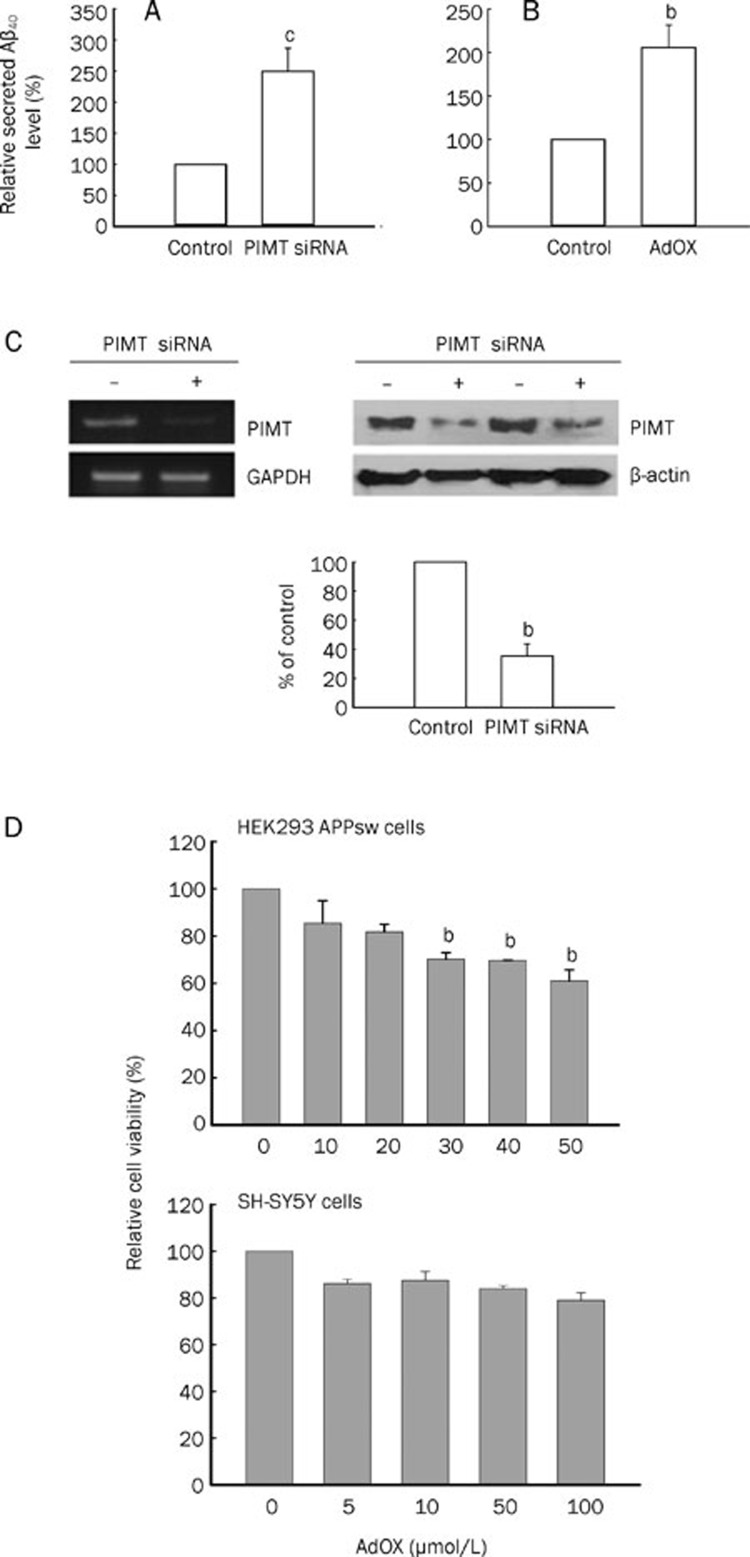
As shown in Figure 1A and 1B, PIMT siRNA transfection and AdOX treatment increased the secretion of Aβ40 in HEK293 APPsw cells approximately two fold. Compared to controls, PIMT siRNA induced a 35.6%±8.0% reduction in mRNA and protein levels in HEK293 APPsw cells 48 h after transfection (Figure 1C). Importantly, the concentration of AdOX used was not cytotoxic (Figure 1D).
Figure 1.
The effects of PIMT siRNA and AdOX treatments on Aβ production. (A and B) HEK293 APPsw cells were treated with or without PIMT siRNA and AdOX. The levels of Aβ40 peptide were analyzed using ELISA. (C) HEK293 APPsw cells were transiently transfected with PIMT siRNA at a concentration of 30 nmol/L. RT-PCR of RNA isolated at 48 h shows PIMT mRNA levels were reduced by PIMT siRNA. As a loading control, GAPDH mRNA levels were examined in HEK293 APPsw cells. Expression levels of PIMT protein were analyzed using a Western blot with rabbit anti-PIMT antiserum. As a loading control, levels of β-actin were also determined. (D) HEK293 APPsw cells and SH-SY5Y cells were treated with AdOX. Cell viability was determined using an MTT assay. Results are expressed as the mean±SD for three independent experiments. Mean±SD. bP<0.05, cP<0.01 compared to untreated or control group.
PIMT siRNA transfection and AdOX decreases sAPPα secretion and increases C99 in HEK293 APPsw cells
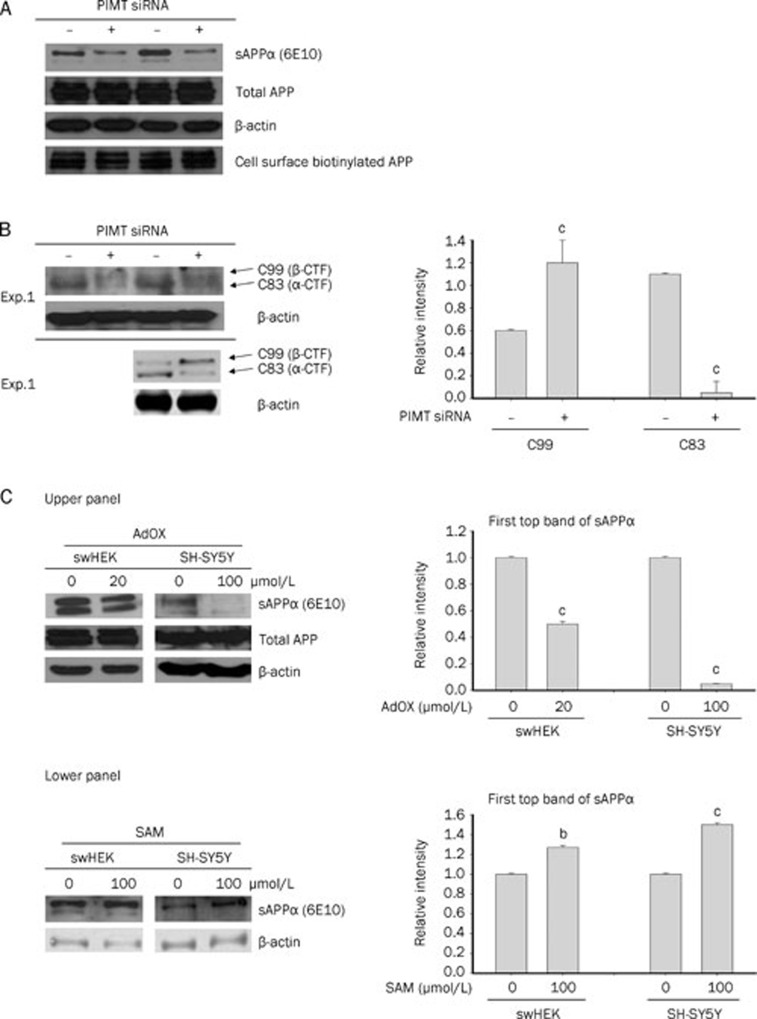
To understand the molecular mechanism of PIMT siRNA-mediated Aβ40 secretion, we evaluated whether PIMT played a role in the processing of APP. To do this, we measured the levels of the APP cleavage products: sAPPα, C99, and C83. Western blotting for sAPPα in the CM revealed that PIMT siRNA decreased the secretion of sAPPα by 67.4%±3.4%. However, the overall expression of total APP and biotin-labeled membrane APP remained unchanged (Figure 2A), suggesting that the amount of sAPPβ, a critical component for secretion of Aβ40, might be increased. Indeed, the C99 fragment, the cleavage product of β-secretase17, was increased in PIMT siRNA-treated cells. At the same time, C83 was decreased in PIMT transfected cells (Figure 2B). Similar patterns of sAPPα and total APP were also observed in the AdOX treatment group (Figure 2C, upper panel). In contrast, the induction of transmethylation with 100 μmol/L SAM, a methyl donor5, increased sAPPα in both cell types (Figure 2C lower panel), demonstrating a critical role of transmethylation in APP processing.
Figure 2.
The effects of PIMT siRNA, AdOX, and SAM treatments on levels of membrane bound APP, total APP, and sAPPα. (A) The accumulation of sAPPα in the concentrated media, total cellular and membrane-bound levels of APP, C83, and C99 were analyzed by Western blot analysis. (B) HEK293 APPsw cells were transiently transfected with PIMT siRNA at a concentration of 30 nmol/L. The levels of C99 and C83 proteins were analyzed using Western blotting. As a loading control, levels of β-actin were also determined. (C) HEK293 APPsw (swHEK) and SH-SY5Y cells were incubated without or with AdOX (20 μmol/L) and SAM (100 μmol/L) for 24 h. The levels of sAPPα in the concentrated media, total cellular APP, and membrane-bound APP were analyzed by Western blot analysis. Mean±SD. bP<0.05, cP<0.01 compared to normal group.
PIMT siRNA transfection decreases ADAM10 and ADAM17 expression
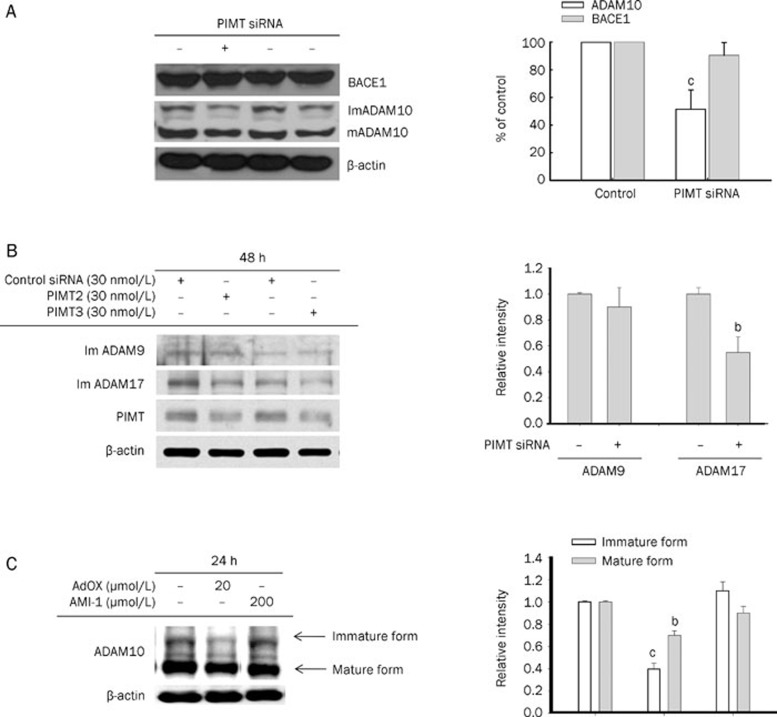
A decrease in sAPPα without a corresponding reduction in total APP expression suggests a potential alteration in the expression or activity of APP processing enzymes. To investigate whether PIMT siRNA altered the expression of APP processing enzymes, we measured the expression levels of several α-secretase candidates: ADAM9, ADAM10, and ADAM17; and the β-secretase candidate, BACE1. As shown in Figure 3A, PIMT siRNA reduced the expression of both the mature and the immature forms of ADAM10 by 45% to 55% compared to controls. In contrast, PIMT siRNA did not significantly alter the levels of BACE1 (90.4%±14% compared to control). PIMT siRNA treatment down-regulated the total protein levels of ADAM17, but not ADAM9 (Figure 3B). In agreement with these data, AdOX, but not AMI-1, a PRMT inhibitor, also strongly reduced ADAM10 levels (Figure 3C). These results suggest that PIMT, but not PRMT, selectively modulates the protein levels of ADAM10 and ADAM17.
Figure 3.
The effects of PIMT siRNA on ADAM10 and BACE1 expression levels. (A) HEK293 APPsw cells were treated with PIMT siRNA for 48 h. Cells were then harvested, and the protein levels of BACE1 and immature (ImADAM10) and mature (mADAM10) forms of ADAM10 were analyzed by Western blot analysis. Densitometric analysis was performed to determine the relative protein level of immature ADAM10 and BACE1. Results are expressed as the mean±SD for three independent experiments. (B) HEK293 APPsw cells were treated with PIMT siRNA for 48 h. Cells were then harvested, and the protein levels of immature ADAM9 and ADAM17, as well as PIMT were analyzed by Western blot analysis. Densitometric analysis was performed to determine the relative protein level of immature ADAM9 and ADAM17. (C) HEK293 APPsw cells were treated with AdOX or AMI-1 for 24 h. Cells were then harvested and the protein levels of immature and mature ADAM10 were analyzed by Western blotting analysis compared to control. Densitometric analysis was performed to determine the relative level of immature and mature ADAM10. Mean±SD. bP<0.05, cP<0.01 compared to normal group.
Discussion
In this study, we used HEK293 APPsw cells and SH-SY5Y cells to investigate the effects of protein L-isoaspartyl methylation on APP processing. HEK293 APPsw cells express high levels of Aβ18, and SH-SY5Y human neuroblastoma cells express considerable levels of APP and secrete non-toxic, non-amyloidogenic sAPP17. Because of this, these cell lines have been widely used to study the regulation of APP processing related to the pathogenesis of AD19. Therefore, we used these cells in our study to examine the regulatory role of transmethylation on APP processing. Interestingly, treatment of either cell type with PIMT siRNA and AdOX, a well-known inhibitor of transmethylation20, remarkably induced the release of Aβ40 peptides (Figure 1), indicating the involvement of PIMT-mediated methylation in APP cleavage. Because numerous papers have shown that the secretion of Aβ40 is accompanied by the release of additional γ-secretase-generated Aβ peptides, such as Aβ38, Aβ42, and Aβ43, it is likely that the production of these peptides would also be regulated by PIMT siRNA treatment.
To investigate the molecular mechanism underlying this phenomenon, the levels of the enzyme that generate Aβ40 peptides were first determined. Figures 2 and 3 reveal that PIMT knock-down modulates both the secretion and cleavage of sAPPα. In response to PIMT siRNA treatment, sAPPα secretion was dramatically diminished, but the levels of total APP and membrane-bound biotinylated APP remained unchanged (Figure 2A). These results suggest that sAPPβ might be relatively enhanced by PIMT siRNA. The C99 fragment, a β-secretase cleavage product of APP, was increased by PIMT knock-down, whereas C83 was dramatically diminished (Figure 2B). Overall, our results suggest that the PIMT-mediated Aβ40 production pathway might be primarily associated with the sAPPα cleavage pathway. It has been reported that green tea polyphenol (–)-epigallocatechin-3-gallate (EGCG) exerts a beneficial role in reducing brain Aβ levels by promoting the cleavage of the C99 fragment of APP. The corresponding elevation of sAPPα21 and G-protein coupled signaling, a major excitatory signal transduction pathway in neuronal cells, is known to activate a sAPPα generation pathway22. Therefore, PIMT knock-down could contribute to the down-regulation of sAPPα during Aβ40 production.
The improper production of APP isoforms or aberrant APP trafficking during AD pathogenesis is believed to favor the amyloidogenic pathway23. In addition, recent reports have shown that Aβ production is influenced more by the location of APP cleavage than the total amount of secretase present within the cell24. However, in our study, neither the total amount of APP nor the amount of membrane-associated APP (Figure 2A) was altered in response to PIMT siRNA treatment. These results imply that PIMT does not regulate the trafficking of APP to the cell membrane or its synthesis. Instead, the protein levels of Aβ40 generating enzymes (Figure 3) clearly reveal an involvement of proteolytic processing in the observed decrease in sAPPα and increase in Aβ40 peptides in response to PIMT siRNA. Indeed, the expression of the α-secretase gene products, α-disintegrin and metalloprotease 10 (ADAM10) and ADAM1721, was reduced after PIMT siRNA transfection in HEK293 APPsw cells according to Western blot analysis (Figure 3). The facts that BACE1 expression was not altered (Figure 3A) and that the PRMT inhibitor, AMI-1, did not affect ADAM10 levels (Figure 3C) seem to highlight the specificity of this pathway leading to Aβ40 generation. However, we cannot exclude the possibility that PIMT knock-down leads to the direct activation of BACE1 despite not affecting its expression level. Indeed, previous work has shown that BACE1-inducible cells exhibit increased production of Aβ40 peptides25. To date, there is no experimental evidence suggesting that PIMT can regulate the enzyme activity of BACE1. However, several studies have reported that BACE1 can be modified by S-palmitoylation26 and ubiquitination27, indicating the importance of post-translational modifications of BACE1. Future studies will determine whether PIMT-induced methylation of BACE1 at aspartyl residues increases its enzyme activity.
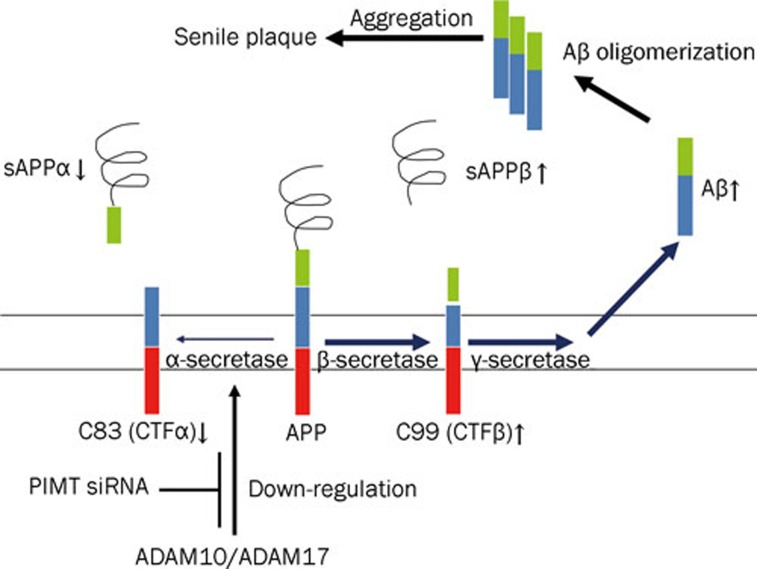
In conclusion, we have demonstrated that knock-down of PIMT increased Aβ production via the inhibition of the non-amyloidogenic α-secretase pathway, an effect that is linked to a decrease in sAPPα and ADAM10/17 levels as summarized in Figure 4. Therefore, our study suggests a novel protective role for PIMT in the pathogenesis of AD as a negative regulator of Aβ40 peptide formation.
Figure 4.
Proposed mechanism of action for PIMT in the regulation of Aβ40 peptide generation. Aβ peptides are generated from APP by β- and γ-secretase activity. PIMT seems to modulate the levels of α- and β-secretases synthesized from ADAM10- and -17.
Author contribution
Narkhyun BAE, Jae Youl CHO, and Sungyoul HONG designed research; Narkhyun BAE, Se Eun BYEON, Jihyuk SONG, and Sang-Jin LEE performed research; Moosik KWON and Inhee MOOK-JUNG analyzed data.
Acknowledgments
This research was supported by Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
References
- Dodart JC, May P.Overview on rodent models of Alzheimer's disease Curr Protoc Neurosci 2005. Chapter 9: Unit 9.22. [DOI] [PubMed]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–90. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–7. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- Grillo MA, Colombatto S. S-adenosylmethionine and its products. Amino Acids. 2008;34:187–93. doi: 10.1007/s00726-007-0500-9. [DOI] [PubMed] [Google Scholar]
- Kennedy BP, Bottiglieri T, Arning E, Ziegler MG, Hansen LA, Masliah E. Elevated S-adenosylhomocysteine in Alzheimer brain: influence on methyltransferases and cognitive function. J Neural Transm. 2004;111:547–67. doi: 10.1007/s00702-003-0096-5. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Sontag JM, Diaz-Arrastia R, Ogris E, Dayal S, et al. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007;27:2751–9. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiglieri T. S-Adenosyl-L-methionine (SAMe): from the bench to the bedside — molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76:1151S–7S. doi: 10.1093/ajcn/76/5.1151S. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Takagi H, Kitamura D, Tatsuoka H, Nakano H, Kawano H, et al. Deficiency in protein L-isoaspartyl methyltransferase results in a fatal progressive epilepsy. J Neurosci. 1998;18:2063–74. doi: 10.1523/JNEUROSCI.18-06-02063.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Watanabe A, Ogawara M, Mori H, Shirasawa T. Isoaspartate formation and neurodegeneration in Alzheimer's disease. Arch Biochem Biophys. 2000;381:225–34. doi: 10.1006/abbi.2000.1955. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Matsuoka Y, Shirasawa T. Biological significance of isoaspartate and its repair system. Biol Pharm Bull. 2005;28:1590–6. doi: 10.1248/bpb.28.1590. [DOI] [PubMed] [Google Scholar]
- Kim CM, Hong WS, Lee JO, Kang TW, Kim YH, Cho CG, et al. A retrospective study on radiotherapy and radiochemotherapy in esophageal cancer. Korean J Intern Med. 1988;3:58–63. doi: 10.3904/kjim.1988.3.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Lee YG, Kim MY, Byeon SE, Rhee MH, Park J, et al. Src-mediated regulation of inflammatory responses by actin polymerization. Biochem Pharmacol. 2010;79:431–43. doi: 10.1016/j.bcp.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier C, et al. Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2010;7:17. doi: 10.1186/1742-2094-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsubo T. Pathogenesis of Alzheimer's disease: implications from amyloid research front. Rinsho Shinkeigaku. 2004;44:768–70. [PubMed] [Google Scholar]
- Okamura N, Arai H, Higuchi M, Tashiro M, Matsui T, Itoh M, et al. Cerebrospinal fluid levels of amyloid beta-peptide1–42, but not tau have positive correlation with brain glucose metabolism in humans. Neurosci Lett. 1999;273:203–7. doi: 10.1016/s0304-3940(99)00644-8. [DOI] [PubMed] [Google Scholar]
- Mills J, Reiner PB. Regulation of amyloid precursor protein cleavage. J Neurochem. 1999;72:443–60. doi: 10.1046/j.1471-4159.1999.0720443.x. [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, et al. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992;360:672–4. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Liu F, Su Y, Li B, Ni B. Regulation of amyloid precursor protein expression and secretion via activation of ERK1/2 by hepatocyte growth factor in HEK293 cells transfected with APP751. Exp Cell Res. 2003;287:387–96. doi: 10.1016/s0014-4827(03)00152-6. [DOI] [PubMed] [Google Scholar]
- Hong S, Heo J, Lee S, Heo S, Kim SS, Lee YD, et al. Methyltransferase-inhibition interferes with neuronal differentiation of P19 embryonal carcinoma cells. Biochem Biophys Res Commun. 2008;377:935–40. doi: 10.1016/j.bbrc.2008.10.089. [DOI] [PubMed] [Google Scholar]
- Obregon DF, Rezai-Zadeh K, Bai Y, Sun N, Hou H, Ehrhart J, et al. ADAM10 activation is required for green tea (–)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 2006;281:16419–27. doi: 10.1074/jbc.M600617200. [DOI] [PubMed] [Google Scholar]
- Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI, et al. P2Y2 nucleotide receptors enhance alpha-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280:18696–702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- Kins S, Lauther N, Szodorai A, Beyreuther K. Subcellular trafficking of the amyloid precursor protein gene family and its pathogenic role in Alzheimer's disease. Neurodegener Dis. 2006;3:218–26. doi: 10.1159/000095259. [DOI] [PubMed] [Google Scholar]
- Massone S, Argellati F, Passalacqua M, Armirotti A, Melone L, d'Abramo C, et al. Downregulation of myosin II-B by siRNA alters the subcellular localization of the amyloid precursor protein and increases amyloid-beta deposition in N2a cells. Biochem Biophys Res Commun. 2007;362:633–8. doi: 10.1016/j.bbrc.2007.08.061. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhou W, Tong Y, He G, Song W. Control of APP processing and Abeta generation level by BACE1 enzymatic activity and transcription. FASEB J. 2006;20:285–92. doi: 10.1096/fj.05-4986com. [DOI] [PubMed] [Google Scholar]
- Cheng H, Vetrivel KS, Drisdel RC, Meckler X, Gong P, Leem JY, et al. S-palmitoylation of gamma-secretase subunits nicastrin and APH-1. J Biol Chem. 2009;284:1373–84. doi: 10.1074/jbc.M806380200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EL, Cameron AN, Piazza F, Walker KR, Tesco G. Ubiquitin regulates GGA3-mediated degradation of BACE1. J Biol Chem. 2010;285:24108–19. doi: 10.1074/jbc.M109.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]