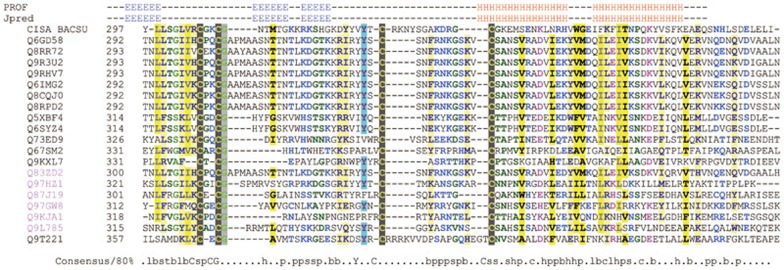
Figure 5.
Alignment of C4 motifs in the serine Int family aligned by ClustalX with manual editing. All of them share a repetitive three β-sheets followed by a HLH structure. A tyrosine is also conserved in these proteins. Q9T221 is the ΦC31 Int derived from Streptomyces. Uniquely, an arginine-rich basic region lies in the centre of the C4 motif of the ΦC31 Int and a conserved HLH structure follows. The alignment is colored and the 80% consensus sequence of the domain calculated using Chroma tool49. Capital letters represent amino acids. Lower-case letters: b, big; h, hydrophobic; l, aliphatic; p, polar; s, small. A secondary structure of this alignment profile is predicted using Jpred50 and PROF51.