Abstract
Aim:
Oncolytic adenovirus, also called conditionally replicating adenovirus (CRAD), can selectively propagate in tumor cells and cause cell lysis. The released viral progeny can infect neighboring cancer cells, initiating a cascade that can lead to the ultimate destruction of the tumor. Suicide gene therapy using herpes simplex virus thymidine kinase (HSV-TK) and ganciclovir (GCV) offers a potential treatment strategy for cancer and is undergoing preclinical trials for a variety of tumors. We hypothesized that HSV-TK gene therapy combined with oncolytic adenoviral therapy would have an enhanced effect compared with the individual effects of the therapies and is a potential novel therapeutic strategy to treat liver cancer.
Methods:
To address our hypothesis, a novel CRAD was created, which consisted of a telomerase-dependent oncolytic adenovirus engineered to express E1A and HSV-TK genes (Ad-ETK). The combined effect of Ad-ETK and GCV was assessed both in vitro and in vivo in nude mice bearing HepG2 cell-derived tumors. Expression of the therapeutic genes by the transduced tumor cells was analyzed by RT-PCR and Western blotting.
Results:
We confirmed that Ad-ETK had antitumorigenic effects on human hepatocellular carcinoma (HCC) both in vitro and in vivo, and the TK/GCV system enhanced oncolytic adenoviral therapy. We confirmed that both E1A and HSV-TK genes were expressed in vivo.
Conclusion:
The Ad-ETK construct should provide a relatively safe and selective approach to killing cancer cells and should be investigated as an adjuvant therapy for hepatocellular carcinoma.
Keywords: conditionally replicative adenovirus, cancer gene therapy, hepatocellular carcinoma, herpes simplex virus thymidine kinase, suicide gene therapy
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. It causes >600 000 deaths annually worldwide and has a very poor prognosis. Despite treatment with surgery, radiotherapy, chemotherapy and ablative therapy (transcatheter arterial chemoembolization, ethanol injection, and radiofrequency ablation), the 5-year survival rate is <20%1, 2. Thus, new therapies are urgently needed for this deadly disease.
Suicide gene therapy using the herpes simplex virus thymidine kinase/ganciclovir (HSV-TK/GCV) system is a well-characterized tool used in cancer gene therapy3, 4, 5, 6, 7. For example, the adenovirus that encodes the HSV-TK gene has been administered to prostate cancer patients and has resulted in some clinical improvement8. Finocchiano et al intratumorally injected lipoplexes that encode HSV-TK and GCV, along with irradiated transgenic xenogeneic cells that secrete human granulocyte–macrophage colony-stimulating factor and interleukin-2 into a spontaneous canine melanoma. They found that repeated injections of the suicide gene system and cytokine-secreting xenogeneic cells into the tumor bed substantially reduced tumor growth, delayed or prevented distant metastasis, significantly extended survival, and improved the quality of life for the dogs9. We propose that the HSV-TK/GCV system combined with other therapies might achieve greater therapeutic efficacy against cancer.
Oncolytic adenovirus, also referred to as conditionally replicating adenovirus (CRAD), can selectively replicate in tumor cells and cause cell lysis. The released viral particles subsequently infect neighboring tumor cells, which leads to tumor lysis and regression. When CRADs infect normal cells, viral replication stops, so they are safe to use in the presence of normal cells10.
Huang et al have constructed a telomerase reverse transcriptase promoter (TERTp)-regulated CRAD for tumor-specific oncolysis by replacing the endogenous adenoviral E1A promoter with human TERTp (Adv-TERTp-E1A). They found that Adv-TERTp-E1A had a cytopathic effect on TERT-positive, but not TERT-negative cells. In a nude mouse xenograft model of human HCC, replication of locally administered Adv-TERTp-E1A increased adenoviral titer in tumor extracts by several orders of magnitude between 6 h and 3 d post injection. Furthermore, Adv-TERTp-E1A treatment significantly inhibited tumor growth and increased areas of tumor necrosis, which stained positively for adenovirus. These effects were not observed with a control replication-deficient adenovirus11. These results indicate that the TERTp-driven CRAD is capable of tumor-selective replication and oncolysis in vitro and in vivo and can be utilized as an adjuvant therapeutic agent for cancer11. Other studies have demonstrated that telomerase-selective oncolytic adenoviral agents significantly suppress cancer growth12, 13, 14.
We hypothesized that combination of Adv-TERTp-E1A with the suicide gene HSV-TK/GCV system would augment anticancer activity in vitro and in vivo. In our study, oncolytic adenoviruses armed with a suicide gene were administered to hepatocellular carcinoma cells and an HCC animal model in order to evaluate the anti-neoplastic efficacy of Ad-ETK alone and to determine whether there was an enhancement when combined with the HSV-TK/GCV system.
Materials and methods
Cell lines and cell culture
The hepatocellular carcinoma cell line, SMMC-7721, a human pancreas carcinoma cell line, MiaPaCa-2, and a human lung adenocarcinoma cell line, GLC-82, were obtained from the Institute of Cell Biology at the Chinese Academy of Sciences, Shanghai, China. The human hepatocellular carcinoma cell line, HepG2 (ATCC No HB-8065), human larynx epidermoid carcinoma cell line, Hep-2 (ATCC No CCL-23), and human normal fibroblast cell line, BJ (ATCC No CRL-2522), were obtained from the American Type Culture Collection (ATCC, Manassas, VA). BJ, MiaPaCa-2, HepG2, and Hep-2 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and glutamine. GLC-82 and SMMC-7721 were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and glutamine. The cells were cultured at 37 °C in a humidified incubator containing 5% CO2.
Construction of viruses
Plasmids pDC515 and AdMax™ backbone pBGHfrtΔE1,3 FLP, pXC1 (containing the E1A fragment) were acquired from Microbix (Canada). Plasmids pcDNA3.1 and LIL-2TKSN (containing the HSV-TK fragment) were gifts from Dr BONAGURO. The plasmid pmRNAIRES-Luc (pmRNA internal ribosomal entry site-luciferase) was previously constructed in our laboratory15. All restriction enzymes, calf intestinal alkaline phosphatase and T4 DNA ligase were purchased from New England Biolabs (Beijing, China). KOD-plus Taq enzyme (Toyobo Shanghai, China) was used for PCR amplification.
Fragments of hTERTp, E1A and HSV-TK were amplified using PCR from HepG2 cells genome, pXC1 and LIL-2TKSN plasmids respectively. The CMV promoter was excised from the plasmid pDC515 by XbaI and EcoRI and replaced with the hTERT promoter. The generated plasmid was called pDChTERTp. The E1A fragment was excised by EcoRI and BamHI and subsequently shuttled into pDC-hTERTp to construct pDC-hTERTp-E1A, which drives E1A expression by hTERTp.
The plasmid pmRNA IRES-Luc was digested with NcoI and SalI to remove the Luc fragment, and this was replaced with the HSV-TK fragment. A new plasmid, pmRNA IRES-HSV-TK, was constructed and digested with BamHI and SalI to obtain an IRES-HSV-TK fragment. Then IRES-HSV-TK fragment was shuttled into pDC-hTERTp-E1A. A new plasmid, pDC-hTERTp-E1A-IRES-TK, was constructed and named pETK. All the fragments that were amplified by PCR were verified by DNA sequencing.
The AdMax™ backbone pBGHfrtΔE1, 3 FLP, and pETK were co-transfected into 293 cells using the calcium phosphate method. The viral progeny were packaged in 293 cells, purified by double CsCl density gradient ultracentrifugation, dissolved in storage buffer (Hanks' buffer, 10% glycerol) and stored at −80 °C. Viral particle (vp) numbers were calculated from the OD260, for which one absorbency unit was equivalent to 1.0×1012 vp/mL, and the final number of plaque-forming units (PFU) was determined by titration on 293 cells under an agarose overlay. The multiplicity of infection (MOI) was calculated from these infectious titers.
PCR-related information and corresponding sites used in the cutting and ligating operation are listed in Table 1.
Table 1. Primers and restriction enzymes.
| Fragment | Primers | Template | GenBank accession No | Restriction enzymes |
|---|---|---|---|---|
| hTERT | 5′-gcg gta cct gcg ctg tcg ggg cca ggc -3′ | HepG2 genome | AF098956 | KpnI |
| 5′-ccg aat tcc acg tgc gcc cac gtg cgc c-3′ | EcoRI | |||
| E1A | 5′-ccg gaa ttc atg aga cat att atc tgc cac g-3′ | pXC1 | AC000008 | EcoRI |
| 5′-cgg gat cct tat ggc ctg ggg cgt tt-3′ | BamHI | |||
| TK | 5′-cat gcc atg gct tcg tac ccc tgc c-3′ | LIL-2TKSN | AB009254 | NcoI |
| 5′-acg cgt cga ctc agt tag cct ccc cca t-3′ | SalI |
RCA (replication competent Ad) assay
HepG2 cells were seeded in 6-well plates and infected with 100 MOI of Ad-ETK. CPE was observed after 4–5 days and cells were harvested. DNA was extracted, and the DNA corresponding to the E1A promoter was detected by routine PCR. DNA isolated from 293 cells was used as a positive control.
The primers used were sense 5′-GGCGTTTTATTATTATAGTCAG-3′ and antisense 5′-TTTCAGTAACGGTGTCGG-3′.
PCR consisted of 30 cycles (94 °C for 30 s, 50.7 °C for 1 min, and 72 °C for 1 min) after an initial denaturation step (95 °C for 1 min). PCR products were analyzed by electrophoresis on 1% agarose gels.
Cell viability assay
Exponentially growing cells were seeded at a density of 3×103 cells per well in 96-well plates. After 24 h, cells were infected with Ad-ETK in 50 μL of culture medium per well at increasing MOIs of 0, 20, 40, 60, 80, and 100, and 50 μL of complete medium containing various concentrations of GCV was co-administered. Cell viability was measured 7 days later by the methylthiazole tetrazolium (MTT) assay (Cell Titer 96 Nonradioactive Cell Proliferation Assay; Promega Madison, Wisconsin, USA), according to the manufacturer's instructions. The medium was changed immediately prior to the assay. Dye solution (20 μL of 5 μg/mL MTT, Sigma-Aldrich) was added to each well and samples were incubated for 4 h in a 37°C tissue culture incubator. The supernatant was discarded and stop solution (150 μL 100% DMSO, Sigma-Aldrich) was added to each well. After the mixture was shaken for 10 min, the color reaction was quantitated by determining the absorbance at 570 nm using an automatic plate reader, with a reference filter of 650 nm. The percentage of cell survival was calculated as the fraction of surviving cells compared with non-infected cells incubated without GCV, which was set as 100%.
Apoptosis assay
Apoptotic cells were detected using FITC-conjugated Annexin-V (Annexin-V-FITC) (Caltag Laboratories, Burlingame, CA) and propidium iodide (PI). Cells were washed twice with cold PBS and resuspended in Annexin-V binding buffer (10 mmol/L HEPES, 140 mmol/L NaCl, 5 mmol/L CaCl2) at a concentration of 1×106 cells/mL. A 100 μL cell solution (1×106 cells) was added to a 5 mL culture tube, and 5 μL of Annexin-V-FITC and 10 μL of PI were added. The tube was gently vortexed and incubated for 15 min at room temperature in the dark. Binding buffer (400 μL) was added to each tube and the cells were analyzed by flow cytometry (Becton Dickinson, Mountain View, CA, USA).
Viral replication in vitro
Exponentially growing cells were seeded at a density of 1×106 cells per well in 6-well plates and infected 24 h later with Ad-ETK at an MOI of 50. After a 2 h incubation, viruses were removed by rinsing twice with PBS, and then 1 mL of fresh medium was added to each well. Cells were collected and combined with the 24- and 48-h post-infection culture supernatant. Lysates were prepared by three freeze-thaw cycles, and the virus was titered using limiting dilution assays on 293 cells.
Animal experiments
Animal experiments were carried out according to the NIH published “Guidelines for the Care and Use of Laboratory Animals” and the institutional guidelines of the Peking University School of Oncology. BALB/c mice were obtained from the Animal Laboratories at the Peking University School of Oncology.
Subcutaneous tumors were established in 6- to 8-week-old BALB/c nude mice (Peking University School of Oncology Laboratories) by a single inoculation of 2×106 HepG2 cells into the dorsal flank of each mouse. After the tumor diameters reached 3–6 mm, the mice were randomly allocated into five groups (physiological saline, GCV, empty adenovirus, Ad-ETK alone, and Ad-ETK combined with GCV) and treated accordingly with drugs and/or adenovectors. Each group consisted of eight mice.
A total of 1×109 PFU adenovector in 100 μL of physiological saline was administered by intratumoral injection once every 2 days to the empty adenovirus (Ad), Ad-ETK alone and Ad-ETK/GCV combination groups, and GCV (20 mg·kg−1·d−1) in 100 μL of saline was injected intraperitoneally once a day into the mice in the GCV and Ad-ETK/GCV combination groups. The adenovector injection was performed 10 times and the GCV injection was performed 20 times. Tumor volume was calculated using the formula a×b2×0.5, where a and b represent the largest and smallest diameters, respectively. The mice were killed when their tumors reached a diameter of 2.0 cm.
RT-PCR analysis of gene expression
Total RNA was extracted from resected tumor tissues using a ToTALLY RNA kit (Ambion, Austin, TX), according to the manufacturer's instructions. Briefly, RT-PCR analysis was performed with 1 μg total RNA and an oligo(dt)-adaptor primer using the OneStep RT-PCR kit (Qiagen, Hilden, Germany). PCR amplification of the E1A gene was carried out for 4 min at 94°C, followed by 30 cycles of 60 s at 94 °C, 60 s at 60°C, and 90 s at 72 °C. PCR amplification of the TK gene was carried out for 4 min at 94 °C, followed by 30 cycles of 60 s at 94 °C, 60 s at 57.2 °C, and 90 s at 72 °C. The PCR primers were as follows: 5′-GAAGATCTGTCATGAGACATATTATCTGC-3′ (sense) and 5′-GGA ATTCTTATGGCCTGGGGCGTTT-3′ (antisense) for E1A; 5′-CAGCAAGAAGCCACGGAAGT-3′ (sense) and 5′-AGCACCCGCCAGTAAGTCAT-3′ (antisense) for TK; and 5′-GGGACCTGACTA ACT ACCTC-3′ (sense) and 5′-CAGTGATCTCCTTCTGCA TC-3′ (antisense) for β-actin.
Western blot analysis
Tumor tissue was lysed in Laemmli's lysis buffer, and lysates were normalized for protein content using the BCA protein assay (Pierce, Rockford, IL). Equal amounts of lysate were separated using 10% SDS-PAGE, and then proteins were transferred onto Hybond Enhanced Chemiluminescence membranes (Amersham Biosciences, Arlington Heights, IL). The membranes were blocked with a blocking buffer containing 5% low-fat milk, PBS, and 0.05% Tween-20 for at a minimum of 2 h or overnight at 4°C. The membranes were washed three times with PBS containing 0.05% Tween-20 (PBST), and then incubated with primary antibodies (HSV-TK: goat polyclonal antibody, sc-28037, Santa Cruz, CA, and E1A: mouse monoclonal antibody, MS-587, Neo Markers, Fremont, CA) for at least 2 h at room temperature. After being washed again with PBST, the membranes were incubated with peroxidase-conjugated secondary antibodies (E1A: goat anti-mouse, sc-2005, Santa Cruz, CA, and HSV-TK: rabbit anti-goat, sc-2768, Santa Cruz, CA), and then blots were developed with a chemiluminescence detection kit (Amersham Biosciences). The expression of total extracellular signal-regulated kinase (ERK) was used as an internal control (first ERK antibody: goat polyclonal antibody, sc-7383, Santa Cruz, CA, and second ERK antibody: rabbit anti-goat, sc-2768, Santa Cruz, CA).
Histological and immunohistochemical staining
The expression of PCNA (proliferating cell nuclear antigen) was detected by immunohistochemical staining. Paraffin sections of tumor were incubated with 30 mL/L H2O2 in methanol at 37 °C for 10 min to quench the endogenous peroxidase activity. The sections were blocked for 20 min at room temperature and then incubated with antibodies against PCNA (Santa Cruz, CA) overnight at 4 °C. The sections were washed with PBS and developed with DAB chromagen.
Statistical analysis
Mouse tumor volume and weight were analyzed using the Mann-Whitney U nonparametric statistical test and P<0.005 was deemed statistically significant. Analysis of the combined effects of Ad-ETK and GCV was performed as described by Chou & Talalay16, 17, 18 using CalcuSyn 2.0 software (Biosoft, Cambridge, UK).
Results
Construction of viruses
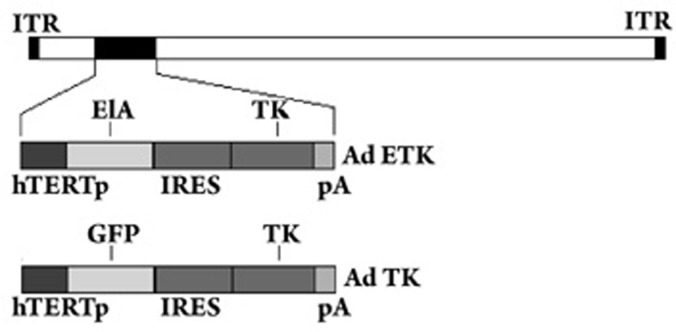
In this study, we aimed to establish a replication-competent adenovirus that expressed the HSV-TK gene and specifically targeted tumor cells. For this purpose, a plasmid containing the E1A gene of Ad5 and the HSV-TK gene was constructed using the pDC515 and AdMax™ backbone pBGHfrtΔE1, 3 FLP recombination system. Figure 1 illustrates the structure of the pETK construct that consists of the hTERT promoter driving the expression of the E1A and HSV-TK genes, which were connected by an IRES. The Ad-ETK and vehicle vector were purified, and the titer was calculated as 6.6×1010 PFU/mL. The E1A fragment in pETK was replaced by an EGFP fragment to construct the pTK plasmid. This adenoviral plasmid expressed HSV-TK but was replication-incompetent.
Figure 1.
Schematic DNA structures of Ad-ETK and Ad-TK. Ad-ETK: the structure of TERTp-regulated conditionally replicative adenovirus, Adv-TERTp-E1A-IRES-TK. The endogenous E1A promoter was replaced with the TERT promoter to control the expression of E1A and TK genes. ITR: inverted terminal repeat. Ad-TK: the structure of TERTp-regulated replication incompetent adenovirus. Adv-TERTp-GFP-IRES-TK: the endogenous E1A promoter was replaced with the TERT promoter to control the expression of GFP and TK genes.
Combination of Ad-ETK and GCV therapy enhanced in vitro cell killing
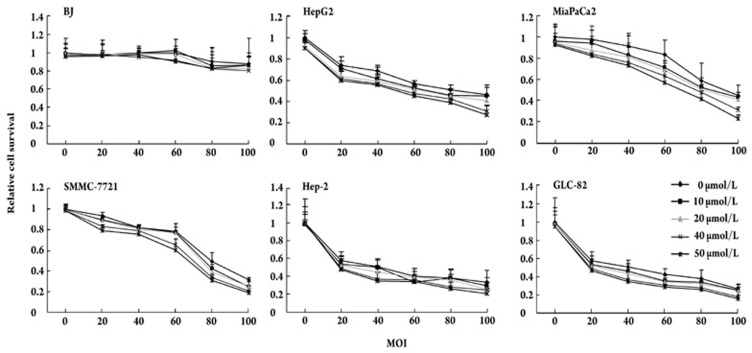
We evaluated the effects of Ad-ETK and GCV combination therapy. To assess the sensitivity of TERT-positive and TERT-negative cells to Ad-ETK and confirm the specific cytotoxicity of the HSV-TK/GCV system, all cells were infected with 0-100 MOI of Ad-ETK and treated with increasing concentrations of GCV (0–50 μmol/L). The number of viable cells was estimated by the MTT assay. As shown in Figures 2 and 3, Ad-ETK and GCV treatment led to dose-dependent killing of TERT-positive cells in vitro and was less toxic to TERT-negative cells.
Figure 2.
Oncolytic effect of combination therapy on TERT-positive cells (HepG2, MiaPaCa2, SMMC-7721, Hep-2, GLC-82) and TERT-negative BJ cells. Cells cultured in 96-well plates were infected by Ad-ETK with serial MOIs and GCV at 0,10,20,40,and 50 μmol/L. The cell survival rate was determined using the MTT assay 7 days post-infection. GCV had minimal deleterious effects on uninfected TERT-positive cell lines at various concentrations, whereas it enhanced the oncolytic effect of Ad-ETK in TERT-positive cell lines. Ad-ETK/GCV had minimal toxicity on the normal human cell line BJ at a high MOI of 100. The assay was performed in quadruplicate, and the results represent the mean±SEM.
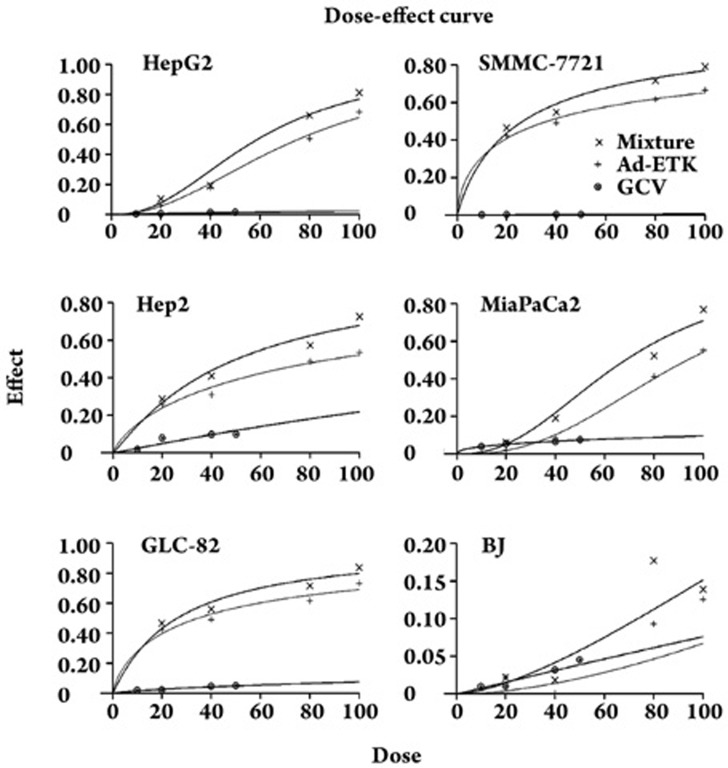
Figure 3.
Cytotoxicity was assessed using the MTT assay and presented as dose-effect curves. Mixture means that Ad-ETK was added to GCV at a constant ratio. In the TERT-positive cells HepG2, SMMC-7721, GLC-82, Hep-2, and MiaPaCa2, Ad-ETK and GCV had a potent synergistic effect on killing cancer cells, whereas there was no effect on the TERT-negative BJ cells.
To further evaluate the effects of Ad-ETK and GCV combination therapy, we determined the combination index (CI) at a constant ratio (the ratio of Ad-ETK and GCV was 2 MOI: 1 μmol/L) in each cell line (Table 2) using CalcuSyn software, which calculated the CI and dose reduction index (DRI). We found that in HepG2, SMMC-7721, Hep-2, GLC-82, MiaPaCa2, and BJ cells, the combination of GCV with Ad-ETK was synergistic (CI<1) at all doses. In TERT-positive and TERT-negative cells, the CI was below 1 for all fa (affected cell fraction) values, indicating synergism for the IC50 to IC90 range with somewhat more synergism (ie, lower CI values) for TERT-positive cells than for TERT-negative cells (Table 2). The dose reduction index (DRI) showed favorable dose reduction for both therapeutics as a result of synergism (Table 2).
Table 2. Combination effects of Ad-ETK and GCV against of TERT-positive and TERT-negative cell lines.
| |
|
Parameters* |
Combination index values at |
DRI† at |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cells | Drugs | Dm | m | r | IC50 | IC75 | IC90 | IC50 | IC75 | IC90 |
| HepG2 | Ad-ETK | 36.12471 | 0.61734 | 0.98733 | 1.521 | 2.408 | 4.225 | |||
| GCV | 6.74×105 | 0.55141 | 0.9578 | 5.90×104 | 1.11×105 | 2.41×105 | ||||
| Ad-ETK +GCV | 26.31734 | 0.90236 | 0.97393 | 0.7285 | 0.4153 | 0.2367 | ||||
| SMMC-7721 | Ad-ETK | 75.23747 | 2.11539 | 0.99396 | 1.279 | 1.324 | 1.37 | |||
| GCV | 13317 | 0.74265 | 0.98722 | 452.73 | 1223.848 | 3308.383 | ||||
| Ad-ETK +GCV | 58.82774 | 2.26584 | 0.97288 | 0.7841 | 0.7562 | 0.7301 | ||||
| MiaPaCa2 | Ad-ETK | 93.52362 | 2.55033 | 0.99753 | 1.368 | 0.319 | 1.272 | |||
| GCV | 22411 | 0.41333 | 0.99184 | 655.706 | 5862.665 | 5.24×104 | ||||
| Ad-ETK +GCV | 68.35834 | 2.35094 | 0.98759 | 0.7325 | 0.7583 | 0.7864 | ||||
| Hep2 | Ad-ETK | 89.1904 | 0.77191 | 0.97625 | 1.776 | 2.678 | 4.039 | |||
| GCV | 339.2943 | 1.04067 | 0.90814 | 13.51 | 14.109 | 14.735 | ||||
| Ad-ETK +GCV | 50.22865 | 1.08529 | 0.96955 | 0.6372 | 0.4443 | 0.3154 | ||||
| GLC-82 | Ad-ETK | 34.50873 | 0.75728 | 0.94948 | 1.323 | 1.936 | 2.833 | |||
| GCV | 4477.363 | 0.65477 | 0.97067 | 343.306 | 630.438 | 1157.721 | ||||
| Ad-ETK +GCV | 26.08383 | 1.02669 | 0.95166 | 0.7588 | 0.5181 | 0.3539 | ||||
| BJ | Ad-ETK | 447.7304 | 1.75568 | 0.51856 | 1.461 | 1.339 | 1.226 | |||
| GCV | 1140.859 | 1.02619 | 0.92895 | 7.447 | 10.643 | 15.21 | ||||
| Ad-ETK +GCV | 306.38 | 1.53967 | 0.87535 | 0.8186 | 0.8411 | 0.8814 | ||||
Dose-effect relationship parameters were calculated on the median-effect equation using a computer software 16. Dm signifies the potency represented by the median-effect dose, which in this case is the concentration (μmol/L) that inhibits the cell growth by 50%. m signifies the shape of the dose-effect curve, where m=1, m>1, and m<1 indicates hyperbolic, sigmoidal, and negative sigmoidal, respectively. r signifies the conformity of data of the method employed, which in this case is the linear correlation coefficient of the median-effect plot.
Combination index (CI) was calculated on the multiple drug effect equation derived by Chou and Talalay 17, 18, using computer software25. CI<1, CI=1, and CI>1 indicate synergism, additive effect, and antagonism, respectively. Dm and m values for single drugs and their combinations were used for CI calculation using the equation Dx=Dm[fa/(1-fa)]1/m and CI=(D)1/(Dx)1+(D)2/(Dx)2, where Dx is the dose (concentration) for x% inhibition. †DRI (dose-reduction index) is measured by comparing the doses required to reach a given degree of inhibition when the drug was used alone and in combination with another drug.
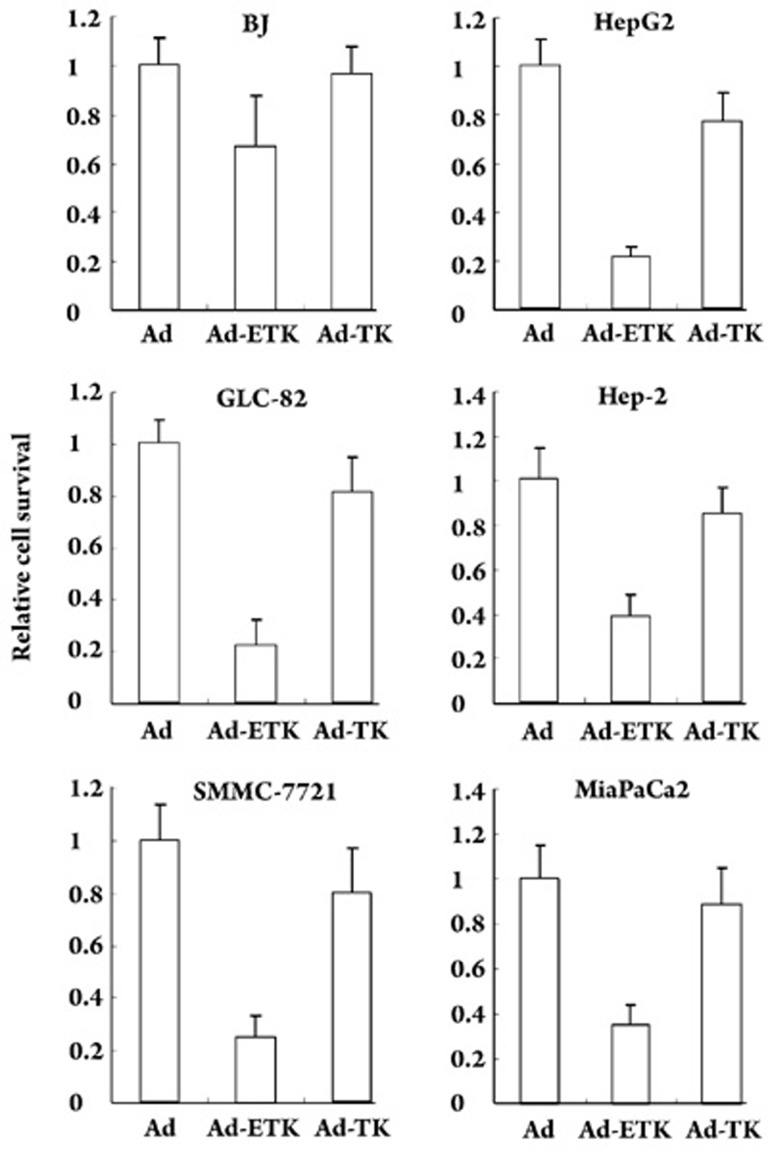
In order to compare the cytotoxity of Ad-ETK, Ad-TK, and Ad, HepG2, SMMC-7721, Hep-2, GLC-82, MiaPaCa2, and BJ cells were infected with Ad-ETK, Ad-TK, and Ad (100 MOI) and exposed to a constant concentration of GCV (50 μmol/L). The number of viable cells was estimated by the MTT assay. As shown in Figure 4, Ad-ETK/GCV had minimal toxicity on the normal human cell line BJ, even at the high MOI of 100, whereas it induced death in approximately 80% of the TERT-positive cells. These data indicate that Ad-ETK selectively kills TERT-positive cells in vitro.
Figure 4.
Comparison of the cytotoxity of Ad-ETK, Ad-TK, and Ad HepG2, SMMC-7721, Hep-2, GLC-82, MiaPaCa2, and BJ cells were infected with Ad-ETK, Ad-TK, or Ad at 100 MOI, and exposed to a constant concentration of GCV (50 μmol/L). The number of viable cells was estimated by the MTT assay.
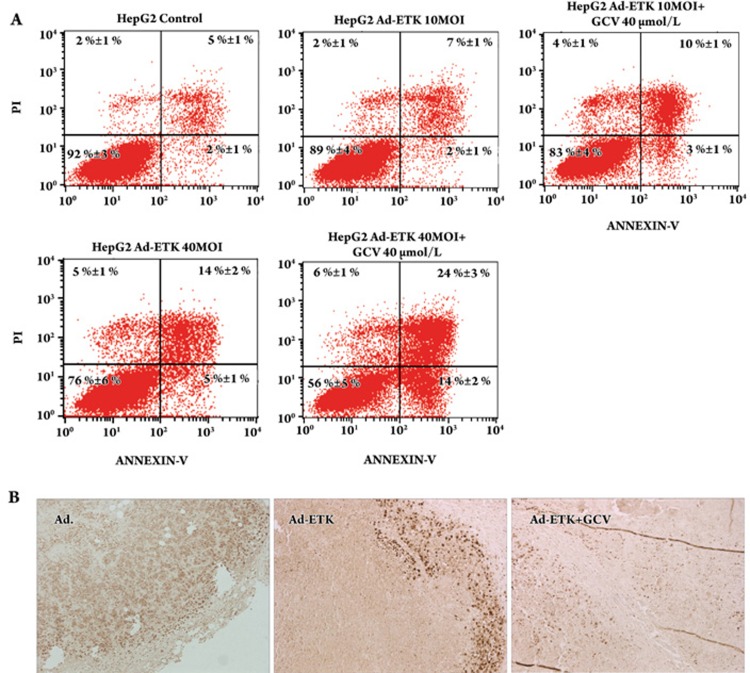
We further evaluated whether the combined effect of Ad-ETK and GCV was significantly greater than that of Ad-ETK alone. We used Annexin V/PI assays to evaluate the combination effect of Ad-ETK and GCV in vitro. As shown in Figure 5A, GCV significantly enhanced the antitumor effects of Ad-ETK.
Figure 5.
Combination effect of Ad-ETK and GCV. (A) The Annexin V/PI assay was used to evaluate the combination effect of Ad-ETK and GCV in vitro. HepG2 cells were treated with different MOIs of Ad-ETK and various concentrations of GCV. After 3 days, cells were assayed. (B) PCNA (proliferating cell nuclear antigen) expression was determined by staining with an antibody against PCNA (10×10).
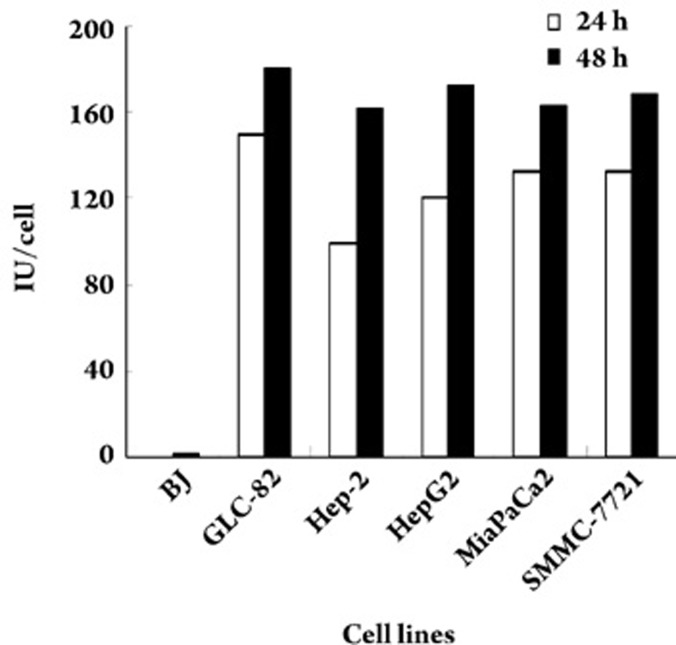
Replication of Ad-ETK in vitro
Various cell lines were infected by Ad-ETK at an MOI of 50, and the viral progeny produced 24 and 48 h after infection were harvested and titrated. As shown in Figure 6, increased viral titer is related to the length of the culture period. For tumor cells, the titer exceeded 100 IU per cell 24 h post-infection and there was significant CPE at 48 h. For the normal cell line BJ, there was a detectable increase in viral titer. The titer was 0.7 IU per cell 48 h post infection, which is much lower than in the tumor cells. This was probably because of the leaky activity of the hTERT promoter; however, the virus could not replicate and spread among the BJ cells. CPE was not observed for BJ cells. These data indicated that Ad-ETK specifically replicated and spread among hTERT positive tumor cells.
Figure 6.
Propagation of Ad-ETK in several human cell lines. Cells were infected by Ad-ETK at an MOI of 50 and then collected together with the culture medium to assay the viral progeny titer at 24 and 48 h post infection. Mean values of duplicates are provided.
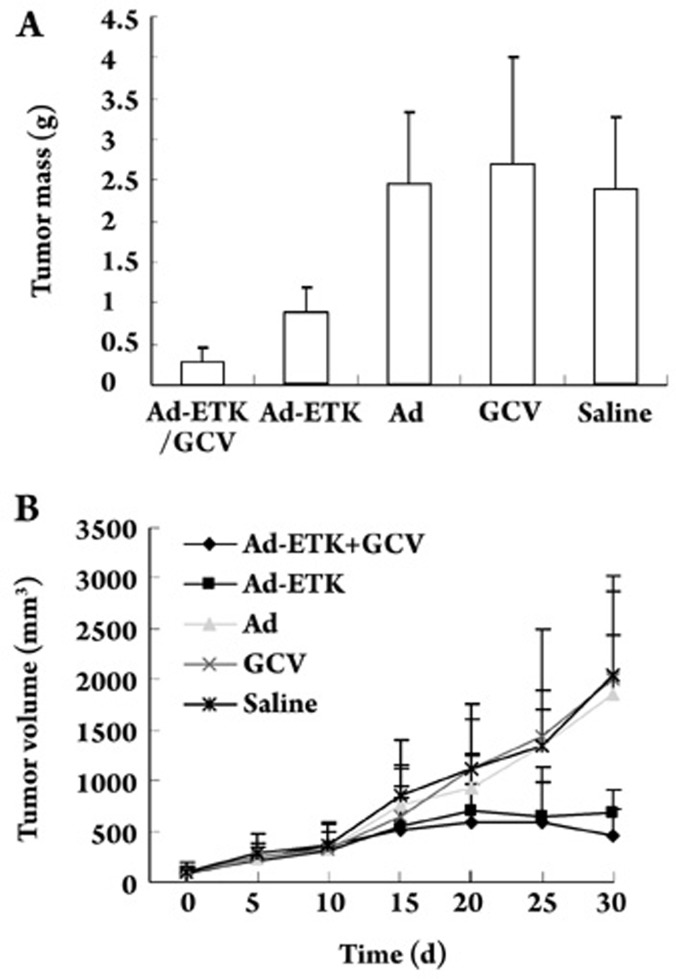
In vivo efficacy studies
All mice were sacrificed on day 30 and all tumors were isolated and weighed. We examined whether GCV combination therapy increased the efficacy of Ad-ETK for treatment of HepG2 xenografts. Treatment with Ad-ETK/GCV or Ad-ETK alone significantly inhibited subcutaneous tumor growth when compared with treatment with adenovirus, GCV or saline (P<0.005). The combination of Ad-ETK and GCV significantly suppressed tumor progression when compared with Ad-ETK or GCV alone (P<0.005). There was no difference in tumor weight after treatment with empty adenovirus, GCV, or saline. Ad-ETK/GCV and Ad-ETK suppressed tumor growth by 74.89% and 50.88%, respectively (Figure 7A).
Figure 7.
Ad-ETK alone and in combination with GCV inhibited HepG2 tumor growth in nude mice. (A) Hepatic carcinoma weight measured 30 days after treatment. The length and width of the tumor were measured every 5 days. All animals were killed when the tumor length in the control group was >20 mm. (B) Growth curve of tumors treated with Ad-ETK/GCV, Ad-ETK, Ad, GCV, or saline. When HepG2 tumors reached 3–6 mm in diameter, the animals were randomly separated into five groups. Data are presented as the mean±SD.
We also analyzed the effect of treatment on tumor volume (Figure 7B). The results did not completely agree with those for tumor mass. There was no significant difference between Ad-ETK alone and Ad-ETK/GCV treatments (P=0.031). There was no difference in tumor volume after treatment with Ad, GCV or saline.
We examined the expression of PCNA in tumor sections by immunohistochemical staining. Ad-ETK dramatically decreased PCNA expression, compared with the high PCNA expression observed in the control group. The expression of PCNA in the Ad-ETK/GCV group exhibited a more significant decrease than in Ad-ETK alone group (Figure 5B).
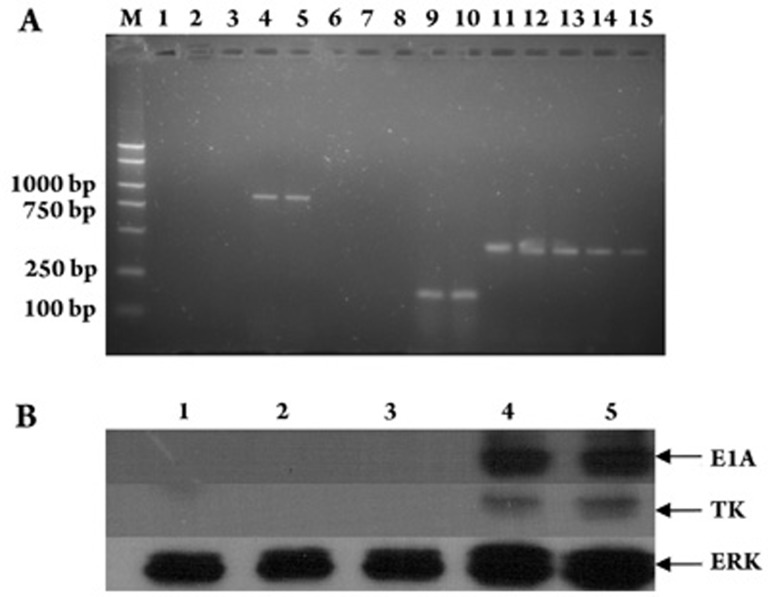
Expression of E1A and HSV-TK genes in tumor tissue
RT-PCR and western blotting were performed to determine the relative expression of E1A and HSV-TK genes in tumors. Electrophoresis of the E1A and TK gene transcripts showed distinct bands at 898 and 173 bp, respectively, in the Ad-ETK and Ad-ETK/GCV combination groups. The other three groups were negative for E1A and TK gene expression (Figure 8A).
Figure 8.
RT-PCR and Western blotting analysis of mouse Ad-ETK transgene and protein expression. (A) RT-PCR was performed on hepatic carcinoma tissue isolated from a mouse infected with Ad-ETK. Lanes: M, 2000 bp DNA maker; 1−5, E1A of saline, GCV, Ad, Ad-ETK, and Ad-ETK/GCV groups, respectively; 6−10, TK of saline, GCV, Ad, Ad-ETK, and Ad-ETK/GCV groups, respectively; 11–15, β-actin of saline, GCV, Ad, Ad-ETK, and Ad-ETK/GCV groups, respectively. (B) Western blotting analysis of E1A and TK expression in tumors. Protein extracted from HepG2 tumors was subjected to Western blotting using antibodies to E1A, TK, and ERK. Lanes: 1, Saline group; 2, GCV group; 3, Ad group; 4, Ad-ETK group; and 5, Ad-ETK/GCV group.
The protein extracted from tumors was subjected to Western blotting using antibodies against E1A, TK and total-ERK. As shown in Figure 5, the Ad-ETK/GCV and Ad-ETK groups had relatively high expression of E1A and TK, whereas the Ad, GCV and saline groups had no expression (Figure 8B).
Discussion
CRADs are attractive anticancer agents because they selectively replicate in tumor cells. Yao Xinglei et al reported that although systemically injected cytomegalovirus (CMV) promoter-driven Ad-HSV-TK lacked any therapeutic effect, mice injected with adenoviral vectors containing TERTp-driven Ad-HSV-TK experienced reduced tumor growth and prolonged survival with minimal side effects. These results suggest that adenoviruses with transgenic expression driven by TERTp are a promising prototype of tumor-targeting vectors for effective and safe cancer gene therapy18. However, as with other gene therapy strategies, targeting with telomerase-specific constructs may be limited by factors such as poor biodistribution and sufficient transgene expression in target tumor cells. CRADs provide an attractive solution because both the biodistribution and the expression of the therapeutic construct increases over time within the target cells.
The selectivity of CRADs is crucial for their tumor-specific targeting. Generally, the selectivity of CRADs for cancer cells is achieved either during the infection or during the replication phases. In the present study, human TERTp was used to direct the expression of the adenoviral E1A and HSV-TK genes, which enabled CRADs to replicate specifically in telomerase-positive tumor cells. We demonstrated that Adv-TERTp-E1A-IRES-TK could kill hepatic carcinoma cells in vitro and in vivo. Previous studies have demonstrated that telomerase-dependent oncolytic adenovirus can inhibit tumor growth11, 12, 13, and our results were in accordance with these studies.
There is controversy regarding the combination of HSV-TK/GCV and replicating adenovirus. Some studies have demonstrated that the antitumor efficacy of HSV-TK-expressing oncolytic adenoviruses is augmented by GCV treatment19, 20, 21. On the contrary, other studies have suggested that GCV does not further improve the oncolytic potential of replicating adenoviruses, at least not in vivo22, 23, 24, 25. The studies by Raki et al and Wilder et al did not assess whether any differences existed in the tumor weight between the groups. They only assessed tumor volume, which did not differ significantly between the CRAD/GCV and CRAD groups. In our study, there was also no significant difference in tumor volume after treatment with Ad-ETK alone and in combination with GCV. However, the tumor weight in the Ad-ETK/GCV group was significantly reduced compared with the Ad-ETK group. There are two possible explanations for this phenomenon: (1) the volume formula (a×b2×0.5) is not accurate for determining the actual tumor volume, and (2) there was extensive tumor liquefaction necrosis, which occurred mainly in the tumors treated with Ad-ETK/GCV and Ad-ETK alone, that were not considered in the tumor mass measurement after dissection.
In addition, there were other issues with the experimental methods used in previous studies that suggested that TK/GCV did not increase the antitumor activity in the context of CRADs. First, the total viral dose used in the Raki study22 might not have been sufficient, because tumor volume in the CRADs group was still increasing when mice were sacrificed at day 29. In our study, we found that the tumor growth in the Ad-ETK/GCV and Ad-ETK groups ceased at day 30. Second, the time of the first injection was too late in the previous studies. Lambright et al25 and Morris et al26 randomized and treated the mice when the mean tumor volume reached ∼200 and 250 mm3, respectively. In our preliminary experiment, Ad-ETK was injected when the mean subcutaneous tumor volume was ∼120 mm3, when there was no difference in tumor weight between the Ad-ETK and Ad-ETK/GCV groups. We presume that the oncolytic effect could not counteract the rapid increase in the number of tumor cells when the tumor volume was >100 mm3. Third, in the study by Lambright et al25, the time of tumor exposure to oncolytic adenovirus (7 days) was not long enough to allow significant morphological changes to develop. Based on these factors, it is inappropriate to conclude from these studies that HSV-TK/GCV did not enhance the antitumor effects of CRADs .
In conclusion, our results clearly indicate that Ad-ETK has both in vitro and in vivo anticancer effects against human HCC, and the HSV-TK/GCV system enhanced oncolytic adenoviral therapy. Our findings suggest that this strategy of using a replication-selective, oncolytic virus is a potentially novel method to treat a variety of human cancers.
Author contribution
Ren-jie YANG, Bin WU, and Qun-wei ZHANG designed research; Fei-qun ZHENG and Yin XU performed research; Xiao-hua TAN constructed virus production; Yi-de QIN contributed new analytical tools and reagents; Fei-qun ZHENG analyzed data and wrote the paper.
Acknowledgments
We thank Prof Jun HAN (National Institute for Viral Disease Control and Preventions, Chinese Center for Disease Control and Prevention) and Zhao-zhang LU, Dong-dong ZHANG for helpful discussions and suggestions.
References
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocelluar carcinoma. Hepatology. 2002;35:519–24. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- Chen SH, Kosai KI, Xu B, Pham-Nguyen K, Contant C, Finegold MJ, et al. Combination suicide and cytokine gene therapy for hepatic matastases of colon carcinoma: sustained antitumor immunity prolongs animal survival. Cancer Res. 1996;56:3758–62. [PubMed] [Google Scholar]
- Kieback DG, Fischer DC, Engehausen DG, Sauerbrei W, Oehler MK, Tong XW, et al. Intraperitoneal adenovirus-mediated suicide gene therapy in combination with either topotecan or paclitaxel in nude mice with human ovarian cancer. Cancer Gene Ther. 2002;9:478–81. doi: 10.1038/sj.cgt.7700462. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Harms JS, Zhu J, Splitter GA. Bovine Herpes virus teguyment protein VP22 enhances thymidine kinase/ganciclovir suicide gene therapy for neuroblastomas compared to herpes simplex virus VP22. J Virol. 2004;78:4224–33. doi: 10.1128/JVI.78.8.4224-4233.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi H, Hayakawa T. Enhanced antitumor effect and reduced vector dissemination with fiber-modified adenovirus vectors expressing herpes simplex virus thymidine kinase. Cancer Gene Ther. 2002;9:236–42. doi: 10.1038/sj.cgt.7700440. [DOI] [PubMed] [Google Scholar]
- Kagaya T, Nakamoto Y, Sakai Y, Tsuchiyama T, Yagita H, Mukaida N, et al. Monocyte chemoattractant protein-1 gene delivery enhances antitumor effects of herpes simplex virus thymidine kinase/ganciclovir system in a model of colon cancer. Cancer Gene Ther. 2006;13:357–66. doi: 10.1038/sj.cgt.7700908. [DOI] [PubMed] [Google Scholar]
- Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F, et al. Suicide gene therapy with adenoviral delivery of HSV-tk gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther. 2007;15:834–40. doi: 10.1038/sj.mt.6300096. [DOI] [PubMed] [Google Scholar]
- Finocchiano LME, Fiszman GL, Karara AL, Glikin GC. Suicide gene and cytokines combined nonviral gene therapy for spontaneous canine melanoma. Cancer Gene Ther. 2008;15:165–72. doi: 10.1038/sj.cgt.7701096. [DOI] [PubMed] [Google Scholar]
- Hu ZB, Wu CT, Wang H, Zhang QW, Wang L, Wang RL, et al. A simplified system for generating oncolytic adenovirus vector carrying one or two transgenes. Cancer Gene Ther. 2008;15:173–82. doi: 10.1038/sj.cgt.7701105. [DOI] [PubMed] [Google Scholar]
- Huang TG, Savontaus MJ, Shinozaki K, Sauter BV, Woo SLC. Telomerase-dependent oncolytic adenovirus for cancer treatment. Gene Ther. 2003;10:1241–7. doi: 10.1038/sj.gt.3301987. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Urata Y, Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Curr Cancer Drug Targets. 2007;7:191–201. doi: 10.2174/156800907780058835. [DOI] [PubMed] [Google Scholar]
- Huang P, Watanabe M, Kaku H, Kashiwakura Y, Chen J, Saika T, et al. Direct and distant antitumor effects of a telomerase-selective oncolytic adenoviral agent, OBP-301, in a mouse prostate cancer model. Cancer Gene Ther. 2008;15:315–22. doi: 10.1038/cgt.2008.3. [DOI] [PubMed] [Google Scholar]
- Uchino J, Takayama K, Harada A, Yosuke K, Hiroyuki I, Curiel DT, et al. Infectivity enhanced, hTERT promoter-based conditionally replicative adenoviruses are useful for SCLC treatment. Cancer Gene Ther. 2005;12:737–48. doi: 10.1038/sj.cgt.7700838. [DOI] [PubMed] [Google Scholar]
- Tan XH, Wan YH. Enhanced protein expression by internal ribosomal entry site-driven mRNA translation as a novel approach for in vitro loading of dendritic cells with antigens. Human Immunol. 2008;69:32–34. doi: 10.1016/j.humimm.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Chou J, Chou TC.Quantification of synergism and antagonism of two or more drugs by computerized analysisIn: Chou TC, Rideout DC, editors. Synergism and antagonism in chemotherapy. San Diego (CA): Academic Press; 1991. p223–244.
- Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem. 1981;115:207–16. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Yao X, Yoshioka Y, Eto Y, Morishige T, Okada Y, Mizuguchi H, et al. TERT promoter-driven adenovirus vector for cancer gene therapy via systemic injection. Biochem Biophys Res Commun. 2007;362:419–24. doi: 10.1016/j.bbrc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Wildner O, Morris JC, Vahanian NN, Ford H Jr, Ramsey WJ, Blaese RM. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999;6:57–62. doi: 10.1038/sj.gt.3300810. [DOI] [PubMed] [Google Scholar]
- Wilder O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–3. [PubMed] [Google Scholar]
- Wilder O, Morris JC. Therapy of peritoneal carcinomatosis from colon cancer with oncolytic adenoviruses. J Gene Med. 2000;2:353–60. doi: 10.1002/1521-2254(200009/10)2:5<353::AID-JGM130>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Raki M, Hakkarainen T, Bauerschmitz GJ, Särkioja M, Desmond RA, Kanerva A, et al. Utility of TK/GCV in the context of highly effective oncolysis mediated by a serotype 3 receptor targeted oncolytic adenovirus. Gene Ther. 2007;14:1380–8. doi: 10.1038/sj.gt.3302992. [DOI] [PubMed] [Google Scholar]
- Wilder O, Morris JC. The role of the E1B 55 kDa gene product in oncolytic adenoviral vectors expressing herpes simplex virus-tk: assessment of antitumor efficacy and toxicity. Cancer Res. 2000;60:4167–74. [PubMed] [Google Scholar]
- Lambright ES, Amin K, Wiewrodt R, Force1 SD, Lanuti1 M, Propert KJ, et al. Inclusion of the herpes simplex thymidine kinase gene in a replicating adenovirus does not augment antitumor efficacy. Gene Ther. 2001;8:946–53. doi: 10.1038/sj.gt.3301489. [DOI] [PubMed] [Google Scholar]
- Morris JC, Wilder O. Therapy of head and neck squamous cell carcinoma with an oncolytic adenovirus expressing HSV-tk. Mol Ther. 2000;1:56–62. doi: 10.1006/mthe.1999.0014. [DOI] [PubMed] [Google Scholar]