Figure 1.
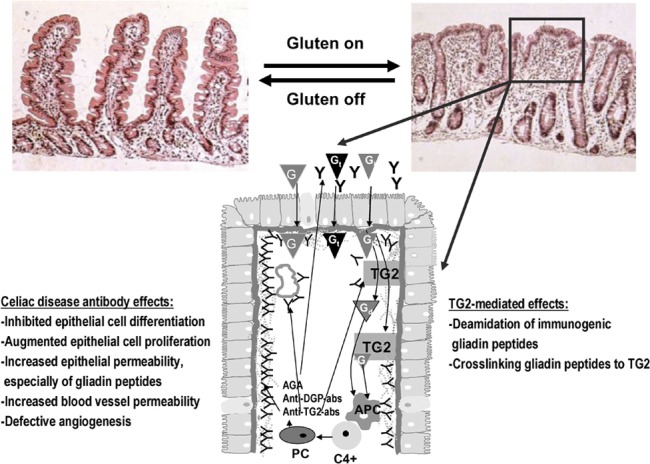
The pathogenesis of celiac disease. In celiac disease patients, the gluten-induced small-bowel mucosal deterioration occurs gradually from normal morphology (shown in the left) to villous atrophy with crypt hyperplasia and inflammation (shown in the right). The schematic presentation (below) shows what happens in the small-bowel mucosa at the cellular level in untreated celiac patients. Gt evoke an innate immunity response, which eventually lead to epithelial cell death and increased epithelial permeability. This enables the Gi to enter the lamina propria where TG2 either deamidates or crosslinks the peptides to themselves. Gd or gliadin-TG2 complexes are taken up by APCs. This is followed by DQ2-/DQ8-dependent activation of CD4+ T cells and the subsequent secretion of antibodies by mucosal PCs. TG2 autoantibodies are found to be deposited in the small-intestinal mucosa below the epithelial basement membrane and around capillaries, but antibodies are also present in the lumen (indicated by Y in the figure). In these distinct locations, the antibodies, which are also targeted against other molecules than TG2, could take part in the disease pathogenesis, and their effects are listed in the figure. When the exogenous trigger gluten is removed, the mucosal damage recovers. AGA, anti-gliadin antibody; anti-DGP-abs, deamidated gliadin peptide antibodies; anti-TG2-abs, TG2 autoantibodies; APC, antigen-presenting cell; G, gliadin; Gd, deamidated gliadin peptide; Gi, immunogenic gliadin peptide; Gt, toxic gliadin peptide; PC, plasma cell; TG2, transglutaminase 2.
