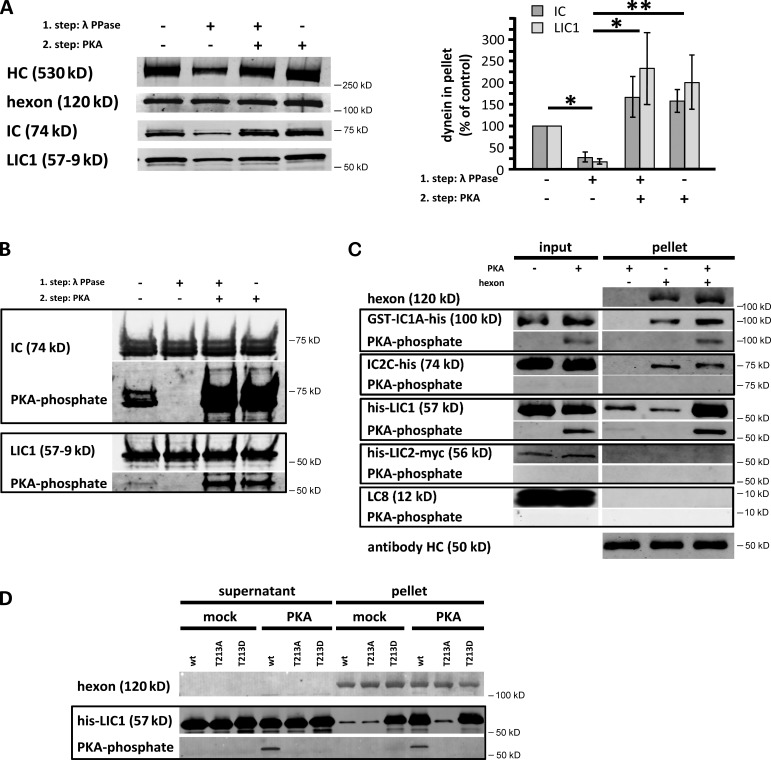
Figure 1.
PKA phosphorylation of the dynein LIC1 subunit controls binding to adenovirus hexon subunit. (A) Purified rat brain cytoplasmic dynein was exposed to λ-phosphatase (PPase) and/or protein kinase A (PKA) catalytic subunit and evaluated for coimmunoprecipitation with hexon by immunoblotting of the pellets with antibodies against hexon, and dynein heavy chain (HC), intermediate chain (IC), and light intermediate chain (LIC) subunits. (right) Quantification of IC and LIC1 immunoblotting results: n = 3, mean ± SD, mock treated = 100%. Dynein dephosphorylation decreased and PKA treatment enhanced binding to hexon. *, P < 0.05; **, P < 0.01 (based on LIC1 signal). (B) Pretreated dynein was evaluated by immunoblotting with antibodies against the dynein ICs, LIC1, and the phosphorylated PKA consensus sequence RRXp(S/T). Clear phosphorylation of the IC and LIC1 subunits is observed. (C) Bacterially expressed full-length dynein subunits IC1, IC2, LIC1, LIC2, and LC8 were exposed to PKA catalytic subunit and tested for phosphorylation and hexon coimmunoprecipitation by immunoblotting with the anti-RRXp(S/T) antibody. LIC1 and IC1 were clearly phosphorylated, which specifically increased LIC1 binding to the hexon. (D) Bacterially expressed LIC1 wild type or the LIC1-T213A and -T213D mutants were tested for PKA phosphorylation and hexon coimmunoprecipitation. The phosphomimetic LIC1-T213D mutant alone interacted with hexon. No PKA phosphorylation was detected in the mutant polypeptides, identifying T213 as the major PKA site. wt, wild type.