Fig. 1.
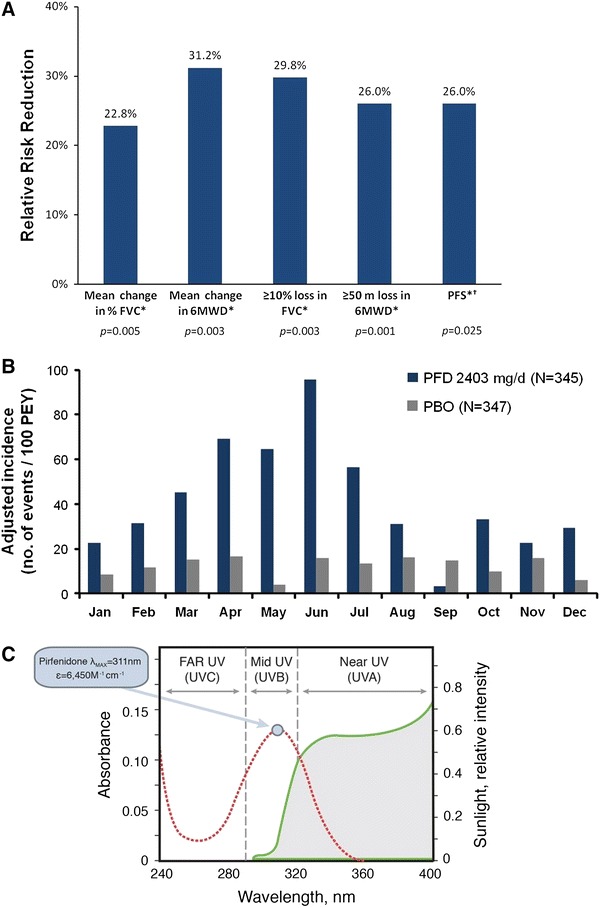
Clinical and preclinical pirfenidone data. a Consistent magnitude of treatment effect with pirfenidone across multiple clinically meaningful outcomes in patients with IPF [15]. Bars represent the magnitude of treatment effect, reported as relative difference between the pirfenidone and placebo groups. *From the pooled analysis of the CAPACITY studies at 72 weeks. †Progression-free survival was defined as time to confirmed ≥10% decline in percentage predicted FVC, ≥15% decline in percentage predicted DLco or death. Deaths were not adjudicated and studies were not powered to evaluate mortality. b The incidence of pirfenidone-related skin rashes in patients with IPF was higher in the early summer months of the CAPACITY studies 004 and 006. Adapted from European Medicine Agency. Pirfenidone CHMP assessment report [38]. c Pirfenidone absorbs light in the ultraviolet spectrum. Adapted from Seto et al. [30]. 6MWD 6-min walk distance, DLco carbon monoxide diffusing capacity, ε molar absorptivity coefficient, FVC forced vital capacity, IPF idiopathic pulmonary fibrosis, λ max wavelength of the most intense ultraviolet absorption, PBO placebo, PEY patient-exposed years, PFD pirfenidone, PFS progression-free survival, UV ultraviolet
