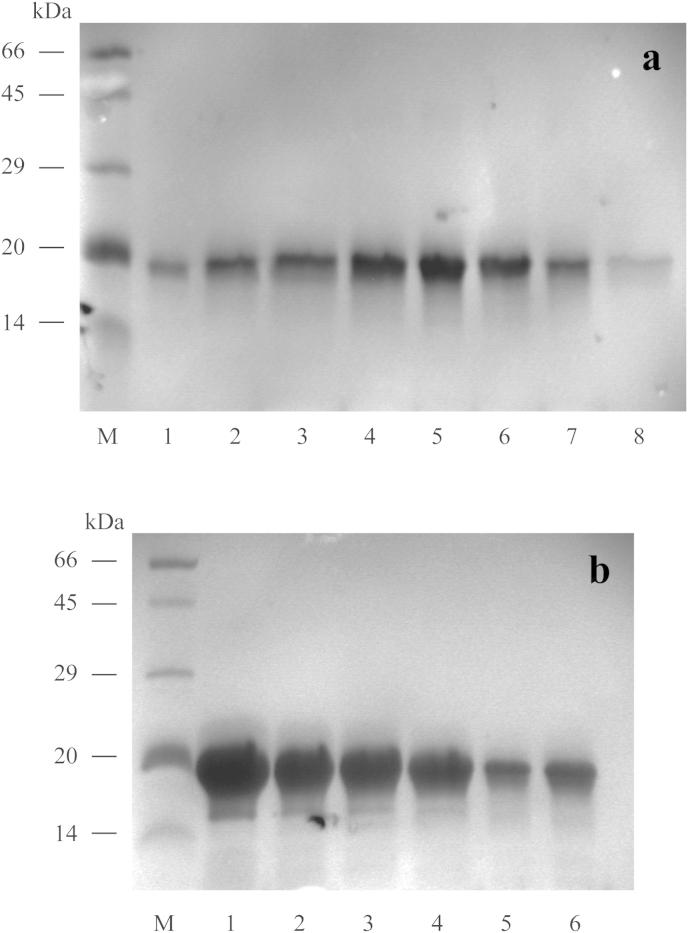
Fig. 6.
SDS-PAGE of purified protein fractions. The fractions related to each peak (Fig. 4, lower panel) were loaded on SDS-PAGE for the first peak related fractions (a) and the second peak related fractions (b). No differences in relation to the molecular weight were identifiable through under denature condition. M: weight marker (BSA: 66 kDa, Ovalbumin: 45 kDa, Carbonic anhydrase: 29 kDa, Trypsin inhibitor: 20 kDa, Lactalbumin: 14 kDa).