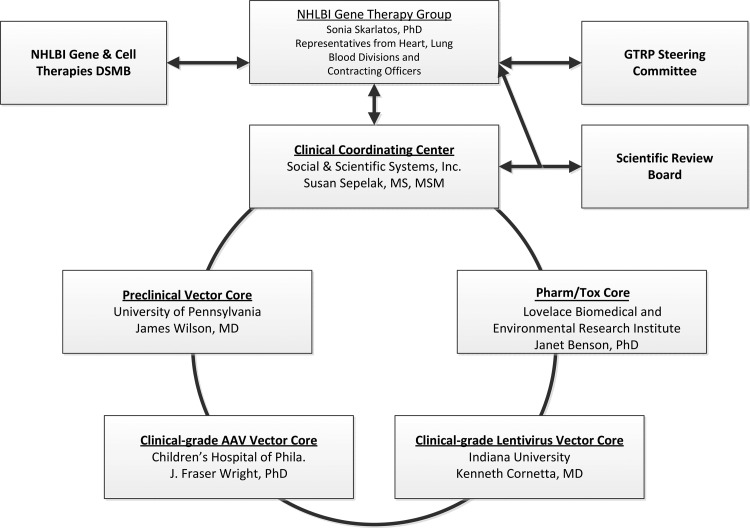
FIG. 1.
Structure and Oversight of the GTRP. The operations of the GTRP are overseen by the NHLBI Gene Therapy Group (GTG) and coordinated by the Clinical Coordinating Center (CCC). The GTG receives program advice from the GTRP Steering Committee (SC) composed of outside experts, ex officio representatives from FDA and NIH Office of Biotechnology Activities (OBA), and the PIs from the Core Laboratories and the CCC. The Scientific Review Board (SRB), a panel composed of experts in various aspects of gene therapy and/or disease-specific fields, provides written reviews on particular funding requests on an ad hoc basis at the request of the GTG. Clinical trials receiving funding assistance from the GTRP are overseen by the NHLBI Gene and Cell Therapies Data and Safety Monitoring Board.