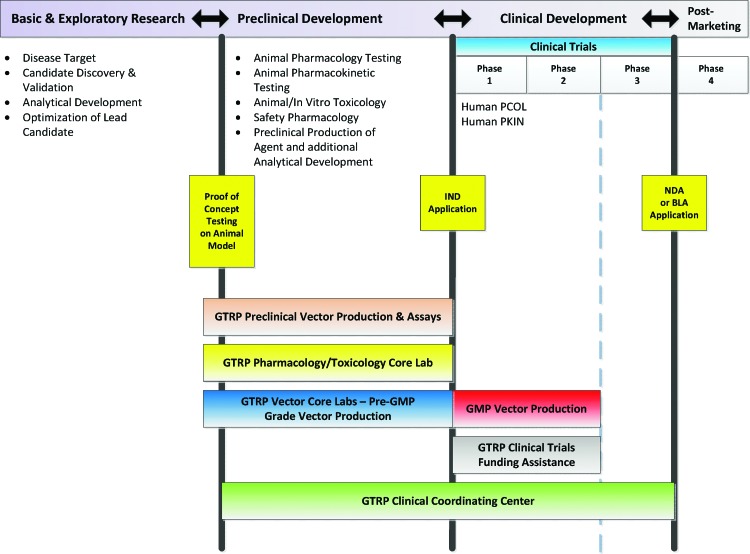
FIG. 2.
Translational Pathway for Investigational Therapeutic Product Development. This figure illustrates the major stages of investigational new drug or biologic development and some of the types of testing that must be done in those stages. The spectrum of coverage by each of the GTRP components is illustrated. In general, the GTRP does not cover the basic and exploratory stage, but some optimization of the lead candidate (i.e., the vector) or analytical development may be conducted by the GTRP laboratories. The GTRP does not fund Phase 3 clinical trials, but the CCC may provide some selected support services. Color images available online at www.liebertpub.com/humc