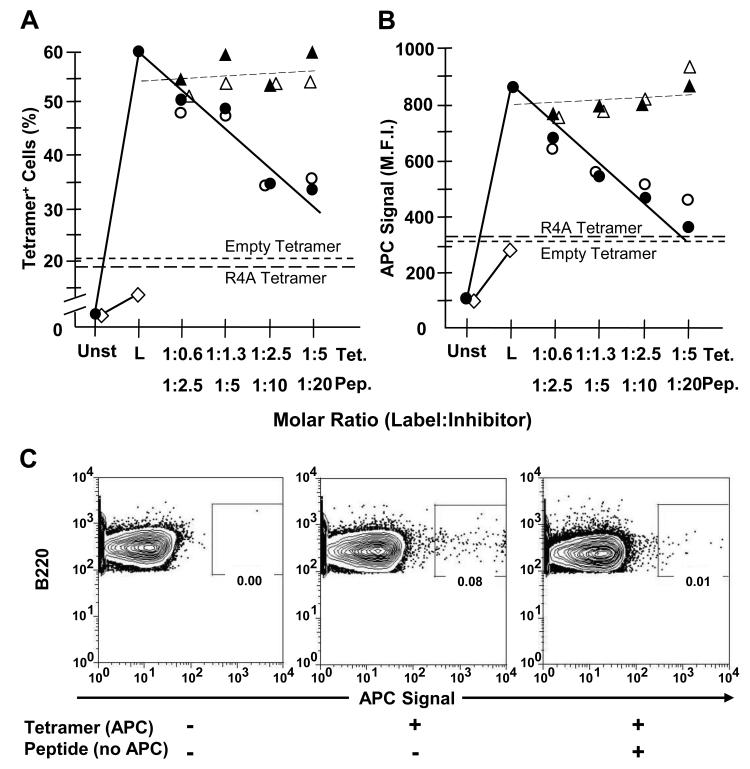
Figure 1. Labeling by MPER-tetramer is blocked by homologous (MPER) but not heterologous (R4A) reagents.
13H11 cells (1-1.3 × 106) were incubated in PBS + 3% FCS containing equivalent molar excess amounts of either unlabeled SP62 (MPER) peptide (○), unlabeled MPER-tetramer (●) or control unlabeled R4A peptide (△) and unlabeled R4A tetramer (▲) for 30 min at 0°C. Unlabeled peptide and tetramer concentrations were established to represent 0.6, 1.3, 2.5 and 5.0 M excess of labeled tetramer-associated peptide epitope. Subsequently, cells were labeled (L) with 125 ng of APC-conjugated MPER-tetramer for 30 min at 0°C. Other 13H11 cells were labeled with either APC-conjugated Empty-tetramer (short dashes) or R4A-tetramer (long dashes) as negative controls for binding. Also, P3 (◇) cells were labeled with APC-conjugated MPER-tetramer as a negative control for binding. Cells were analyzed by FACS and (A) fraction of tetramer-binding cells and (B) M.F.I. of tetramer+ cells was determined. Each data point represents the average of at least 3 independent measurements (n≥3) compiled over 2 independent experiments. (C) BL/6 BM cells (106) were incubated alone or with 10-fold molar excess of MPER peptide. Then, BM cells were incubated alone or labeled with 125 ng of APC-conjugated MPER-tetramer. All samples were washed and labeled with mAb to B220. FACS plots are pre-gated on live, single, B220+ cells. Data are representative of 2 independent experiments.