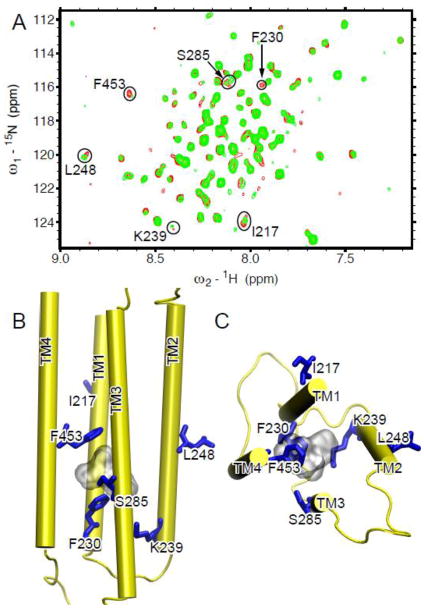
Fig. 5. Ketamine binding site in the TM domain of the human α7 nAChR.
(A) Overlay of 1H-15N TROSY-HSQC spectra of α7 in the absence (red) and the presence (green) of 80 μM ketamine. Residues involved in ketamine binding demonstrated significant changes in chemical shift or peak intensity. They are highlighted in circles and labeled with the one-letter amino acid code and the sequence number. (B) Side and (C) top views of the α7 structure highlighting the residues (blue sticks) perturbed by ketamine (gray surface) binding.