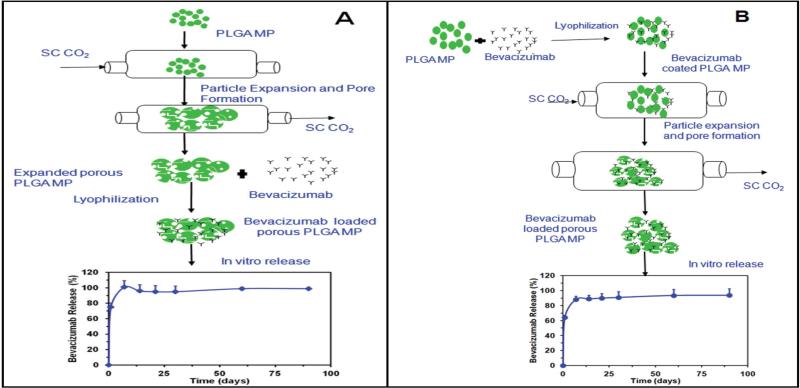
Figure 1.
Schematic representation for the preparation of bevacizumab encapsulated porous PLGA microparticles prepared by supercritical pressure quench technology. (A) Plain PLGA microparticles were made porous by supercritical pressure quench technology and bevacizumab was loaded (PMP1). (B) Bevacizumab was coated on plain PLGA microparticles and exposed to supercritical CO2 (PMP2). The release was performed in PBS pH 7.4 and bevacizumab content was estimated using a micro-BCA kit. Results are expressed as mean ± SD for n=3.