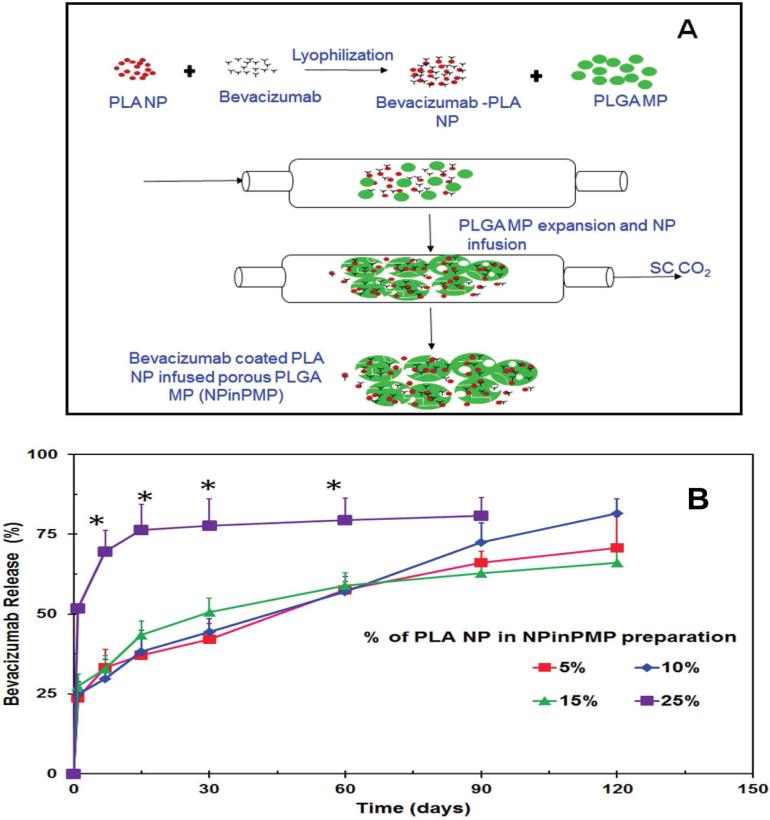
Figure 2.
(A) Schematic representation for the preparation of bevacizumab coated PLA NP infused porous PLGA MP (NPinPMP) using supercritical fluid technology. Bevacizumab coated PLA nanoparticles (NP) were lyophilized and mixed with plain PLGA microparticles (MP) and treated with SC CO2 at 1200 psi/33 °C for 30 min. (B) In vitro cumulative release of bevacizumab from NPinPMP formulations. The release was performed in PBS pH 7.4 and bevacizumab content was estimated using a micro-BCA kit. Results are expressed as mean ± SD for n=3.