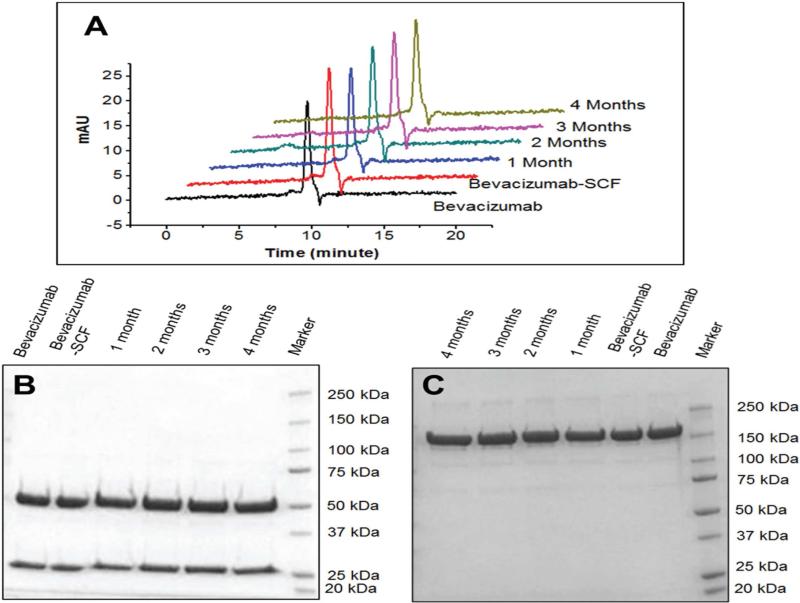
Figure 4.
Structural stability evaluation of bevacizumab by size exclusion chromatography and SDS-PAGE. (A) SEC chromatograms of native, SC CO2 treated bevacizumab and in vitro release samples of bevacizumab from NPinPMP formulation. (B) Reducing, and (C) Non-reducing gel SDS-PAGE pictures of native, supercritical CO2 treated, and in vitro release samples of bevacizumab after 1, 2, 3, and 4 months.