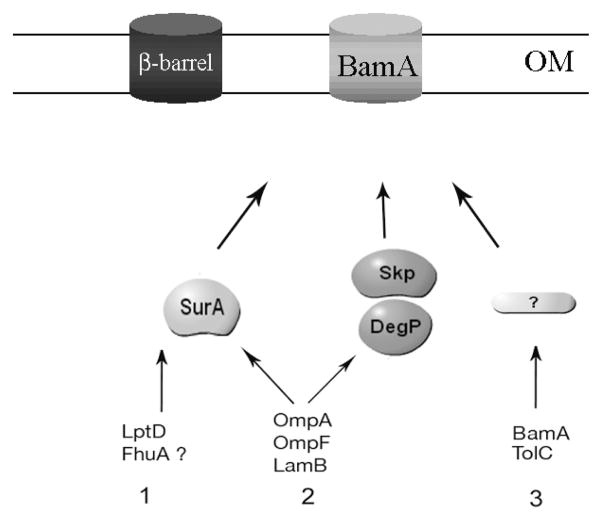
Figure 5. A refined model for SurA’s function in the E. coli periplasm.
Unfolded β-barrel proteins are transported across the IM by the Sec translocon. After cleavage of the signal sequence, they are escorted across the periplasm by periplasmic chaperones before reaching the OM where they are inserted by the Bam complex [1]. There are 3 known periplasmic chaperones that interact with OMPs: SurA, Skp and DegP. Our results indicate that only a subset of OMPs strongly depend on SurA for biogenesis. We propose to divide the OMPs in three groups. Group 1 includes β-barrel proteins, such as LptD and possibly FhuA, that greatly depend on the presence of SurA for biogenesis. Group 2 includes β-barrel proteins such as the major OMPs OmpA, OmpF and LamB that preferentially interact with SurA for biogenesis but are also able to interact with the other periplasmic chaperones. Group 3 includes β-barrel proteins such as BamA and TolC that do not seem to require the presence of SurA for biogenesis. Werner et al. have shown that the assembly of TolC does not depend on Skp and DegP [37] and the fact that a skPdegP double mutant is viable suggest that these two chaperones are not required for the assembly of the essential protein BamA. BamA and TolC are therefore likely to depend on other periplasmic folding factors that are yet to be identified.