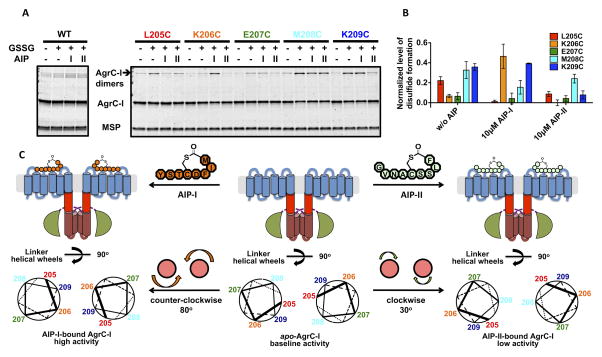
Figure 6. Effect of AIP binding on conformation of the AgrC-I linker helices, see also Figure S6.
(A and B) Cysteine crosslinking of AgrC-I protomers bearing single cysteine point mutants in the TMH-DHp linker region: nanodiscs incorporated with wild-type or mutant AgrC-I dimers were mock-treated or incubated with 10 mM oxidized glutathione (GSSG) along with indicated AIP. (A) Formation of covalent dimers as analyzed by SDS-PAGE with CBB stain. (B) Quantification of the crosslinking results. Plot shows normalized levels of disulfide-linked dimer formation, grouped according to the three ligand states (see Experimental Procedures). Error bars = SD (n = 3). (C) Rotation of the TMH-DHp linker helix in AgrC-I induced by AIP binding. Top: Cartoons of AgrC-I dimer in the apo- (middle), AIP-I-bound (left) and AIP-II-bound (right) states. Bottom: The corresponding conformation of the linker helices is depicted with helical wheels showing the positions 205-209 according to the cysteine crosslinking results. Ligand binding and the consequent rotation of linker helices are highlighted between panels.