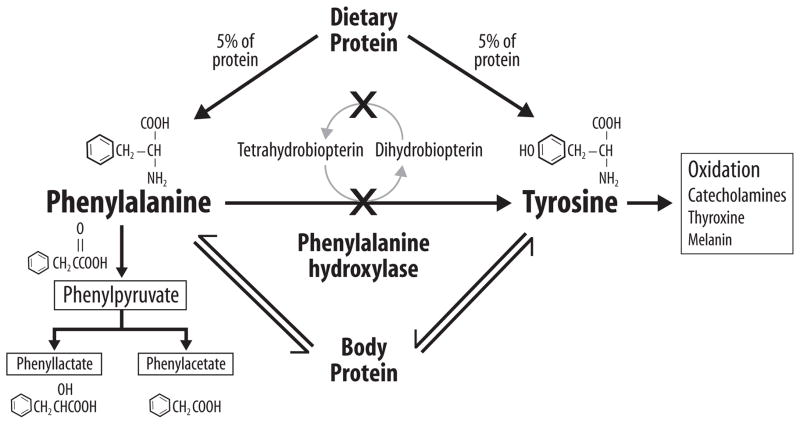
Figure 1.
Phenylalanine (phe) metabolism in phenylketonuria (PKU). As indicated by the “X”, PKU results from mutations (over 800 have been identified) that affect the hepatic phe hydroxylase (PAH) enzyme needed for the hydroxylation of the indispensable amino acid phe to tyrosine. PKU may also result from mutations in genes involved in recycling the essential PAH cofactor tetrahydrobiopterin. Due to these mutations which reduce the conversion of phe to tyrosine, phe accumulates in blood and is transaminated and decarboxylated into many compounds which appear in blood and urine; three of the compounds which are measured clinically are shown. Tyrosine, a precursor for multiple biological products, becomes an indispensable AA and must be provided by the diet for those with PKU. Under physiological conditions PAH catalyzes about 75% of the phe input from the diet and protein catabolism.