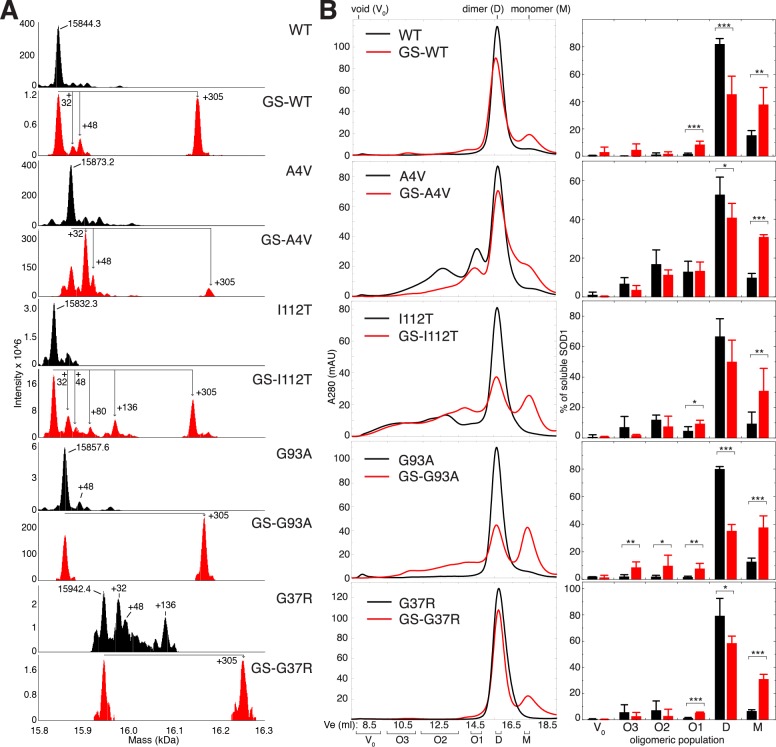
Figure 2.
Cys-111 glutathionylation that occurs endogenously promotes the formation of non-native apo-SOD1 oligomers. (A) Analysis of full-length SOD1 by mass spectrometry. For each SOD1 variant studied, deconvoluted mass spectra are shown for two populations separated by ion exchange chromatography: one in which unmodified SOD1 is the predominant species (black spectra) and one enriched in post-translationally modified SOD1 (red spectra). Labeled masses correspond to the average masses obtained by the deconvolution of spectra using ProMass for Xcalibur software. Mass shifts consistent with glutathionylation (+305), irreversible oxidation (+32, +48), and phosphorylation (+80) are labeled. Occasionally, a mass shift of +136 was observed and is consistent with SOD1 containing phosphorylation (+80), oxidation (+32), and a nonspecific sodium adduct originating from the sample buffer (+22), all of which have been previously observed on SOD1.8,10 (B) Left, SEC chromatograms showing populations of soluble oligomers of unmodified (black) and glutathionylated (red) apo-SOD1 incubated at 100 μM (initial dimeric concentration) for 1 week at 37 °C in 20 mM Tris and 150 mM NaCl at pH 7.4. Right, oligomeric populations quantified by deconvolution of SEC data and integration of Gaussian curves corresponding to individual oligomeric populations. Bar heights represent average values, and error bars represent SD from at least 3 independent experiments. Student’s t test was used to compare the abundance of oligomers in the presence and absence of Cys-111 glutathionylation. * = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001.