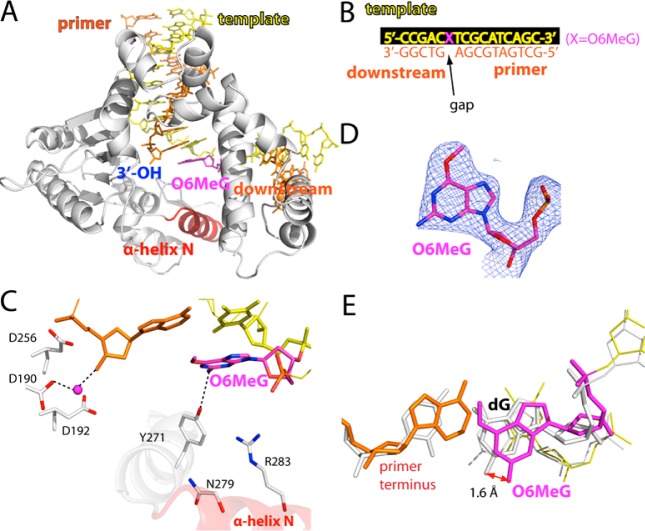
Figure 2.
Structure of polβ bound to DNA containing a single-nucleotide gap opposite templating O6MeG (PDB ID 4MF2). (A) Overall structure of the gapped pol β complex. (B) DNA sequence used for crystallization of the O6MeG gapped complex. The O6MeG·C/T ternary complex structures have dCTP or dTTP analogue opposite templating O6MeG. (C) Active-site view of the gapped structure. Protein is in an open conformation. The three aspartic acid residues as well as Tyr271, Asn279, and Arg283 are indicated. H-bonding interactions are indicated as dotted lines. An ordered water molecule is depicted as a magenta sphere. (D) A 2Fo – Fc map contoured at 1σ around O6MeG lesion. (E) Structural overlay of the templating base and primer terminus in the O6MeG gapped binary complex and published G gapped binary complex35 (PDB ID 1BPX).