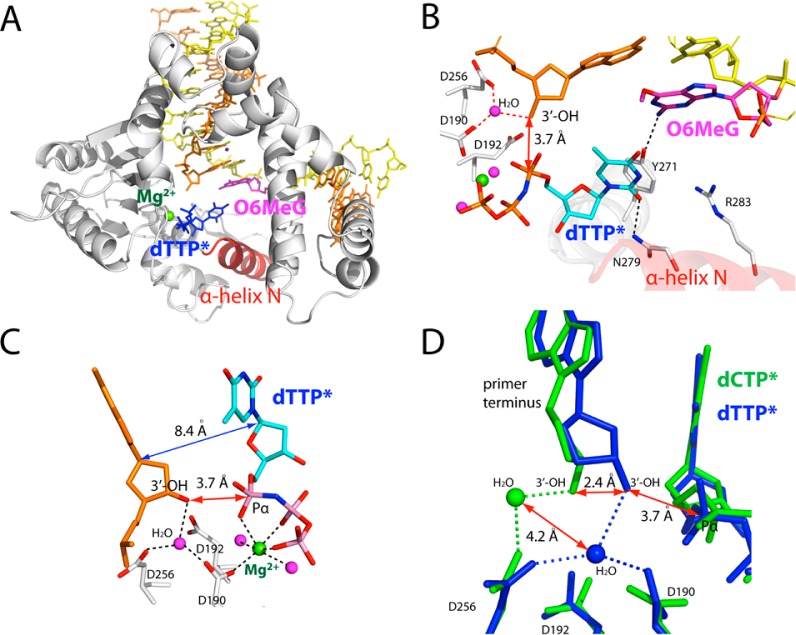
Figure 4.
Ternary structure of polβ incorporating a nonhydrolyzable dTTP analogue (dTTP*, shown in cyan) opposite templating O6MeG in the presence of Mg2+ (PDB ID 4MFF). (A) Overall structure of the O6MeG·T–Mg2+ ternary structure. (B) Active-site view of the O6MeG·T–Mg2+ ternary structure. Protein is in an open conformation. O6MeG and dTTP* form a staggered base pair. Ordered water-mediated H-bondings not observed in the O6MeG·C–Mg2+ ternary structure are indicated in red dotted lines. (C) Close-up view of the metal-ion-binding site. Only nucleotide-binding metal ion is present in this structure. An ordered water molecule that bridges Asp256, Asp190, and primer terminus 3′-OH replaces the catalytic metal ion observed in polβ ternary structure. (D) Overlay of the metal-ion-binding site of the O6MeG·C–Mg2+ structure (green) and the O6MeG·C–Mg2+ structure (blue). Note differences in the positions of the primer terminus 3′-OHs and ordered water molecules. The 5′ side of the primer terminus base is omitted for clarity.