Table 1. Binding Constants of Selected and Nonselected Glycopeptides.
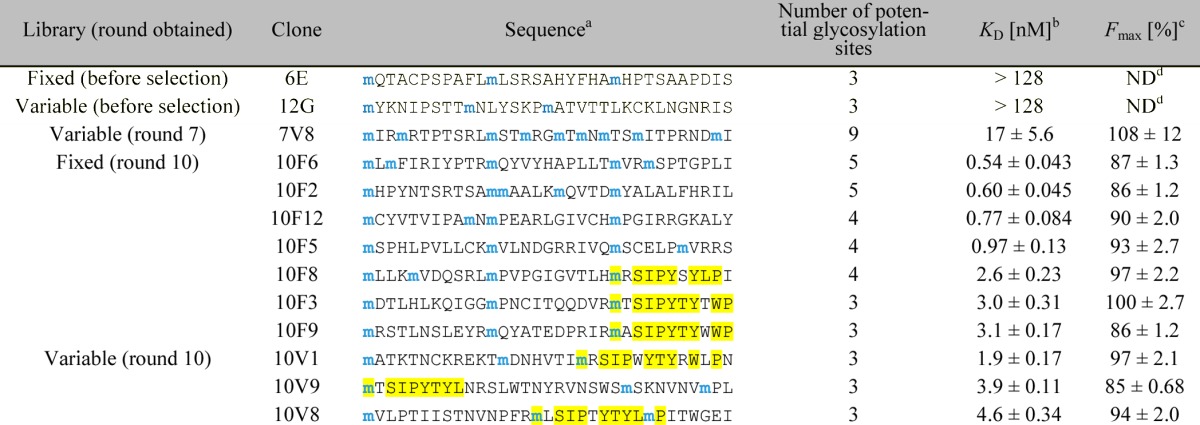
Only the sequence of the random region (positions 1–33) is shown. All peptide sequences used in the 2G12-binding assay were followed by a linker, a His6-tag, and a FLAG-tag (GSGSLGHHHHHHRDYKDDDDK) for purification and radiolabeling purposes. Blue “m” denotes potential Man9-glycosylation sites encoded by the AUG codon. The observed consensus motif is highlighted in yellow.
In the assay, the peptides were radiolabeled with 35S-cysteine (for peptides containing cysteine) or 3H-histidine (for peptides not containing cysteine), and incubated with various concentrations of 2G12, and 2G12–peptide complexes were isolated with magnetic protein G beads. Percentages of the fractions bound were calculated from radioactivity measured by liquid scintillation counting (see Experimental Section for details). KD and Fmax (maximum fraction bound) were calculated by fitting Fbound = (Fmax [2G12])/(KD + [2G12]) to average data points. Errors reported are the standard error of the curve fit.
Not determined.
