Figure 2.
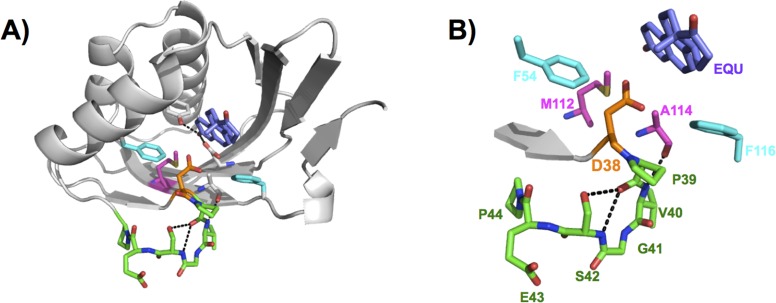
General base, Asp38, positioned in the KSI active site. (A) Superposition of the WT unbound structure (PDB entry 8CHO) and the equilenin-bound D38N structure (PDB entry 1QJG), where only equilenin (EQU) is shown from the equilenin-bound structure. The Asp general base (orange) is located at the end of a β-strand (gray) and at the beginning of a loop (green), and it is situated near Phe54 and Phe116 (cyan) and Met112 and Ala114 (magenta). The bound equilenin ligand (blue-violet) is situated at the location of the bound substrate during the isomerization reaction. (B) Detailed view of the general base and surrounding residues with labels. Colors are as in panel A.
