Figure 6.
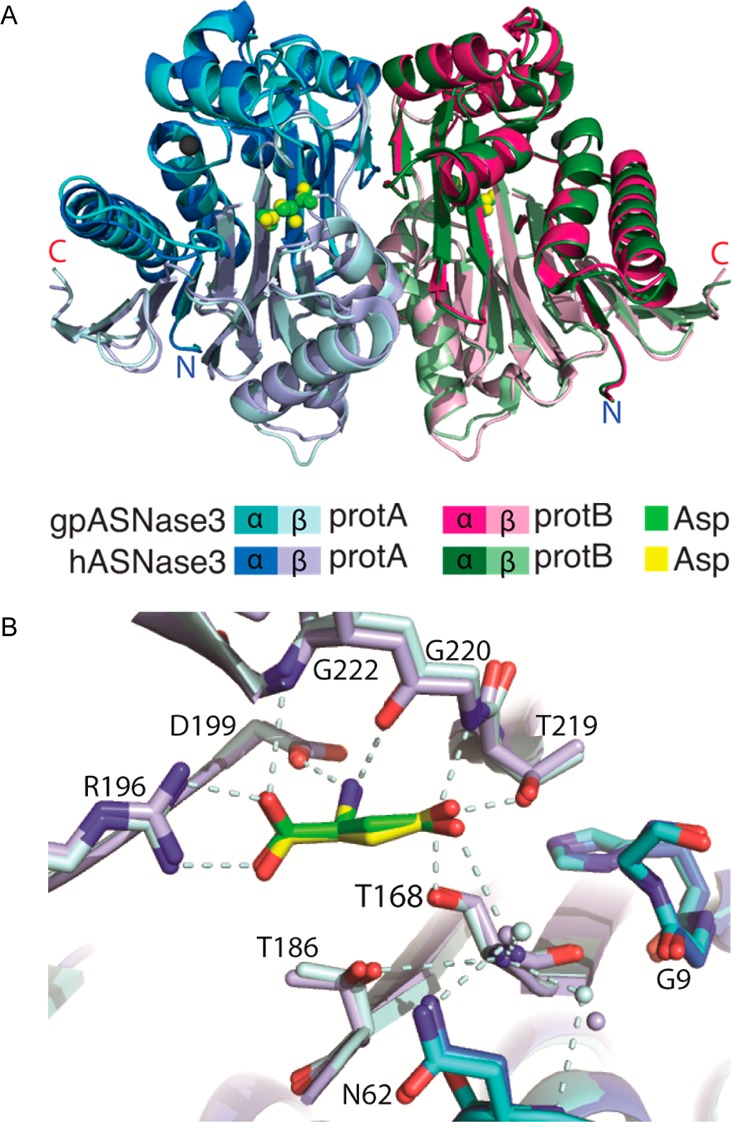
Comparison of the structures of cleaved guinea pig and human ASNase3. (A) Overlay of cartoon diagrams of the structures of cleaved gp and hASNase3 (PDB entry 4GDW) in complex with Asp. The Na+ cation present in each protomer is represented as a gray sphere. (B) Overlay of the active sites of cleaved gp and hASNase3 in complex with Asp denoted in green and yellow, respectively. The freed N-terminus at Thr168 denoting cleavage into α- and β-subunits is observed. Interactions made by conserved Ntn-hydrolase residues (dashed light cyan lines) are consistent between gp and hASNase3. The carbonyl of Gly9 in the HGG loop is also pointed away from the side chain of His8 in both gp and hASNase3 in agreement with cleavage between Gly167 and Thr168 and enzyme activation. Water molecules are shown as small spheres whose colors correspond to either h or gpASNase3.
