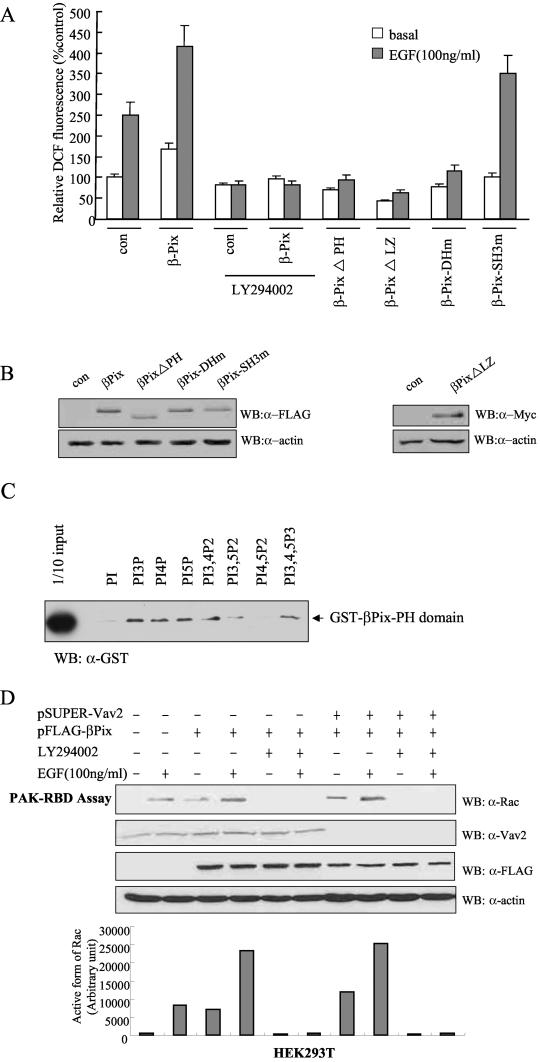
FIG.3.
Roles of the PH domain and LZ motif of βPix in EGF-induced ROS generation. (A) Caco-2 cells were transfected with empty vector (pFLAG-CMV, or pcDNA3.0 for Myc epitope-tagged βPix-ΔLZ) or with vectors encoding either wild-type βPix or βPix mutants. They were then deprived of serum for 16 h and incubated for 10 min in the absence or presence of EGF (100 ng/ml). Cells were pretreated with LY294002 (10 μM) for 30 min before the treatment with EGF (100 ng/ml). The generation of H2O2 was assayed on the basis of DCF fluorescence. Data are means ± SE of values from five independent experiments. (B) Lysates of the transfected cells were subjected to immunoblot analysis with antibodies to the FLAG or Myc epitope, as indicated. (C) Phosphoinositide binding assay. Purified GST-βPix-PH domain (∼200 ng) was incubated with phosphoinositide analogue beads (Echelon Research Laboratories Inc.) overnight at 4°C and then washed with PBS containing 1% Triton X-100. Bead-bound proteins were separated by SDS-PAGE and analyzed by immunoblotting with antibodies against GST. (D) HEK293T cells were transfected with FLAG-βPix only or together with pSUPER-Vav2. After being cultured for 48 h, the cells were deprived of serum for 12 h. The cells were pretreated with LY294002 (10 μM) for 30 min before a treatment with EGF (100 ng/ml). After EGF treatment for 10 min, cell lysates were then prepared in PBS containing 1% Triton X-100 and were incubated for 3 h with a GST fusion protein of PAK-RBD conjugated to glutathione-Sepharose 4B beads. The beads were then washed with ice-cold cell lysis buffer, and bound proteins were subjected to immunoblot analysis with antibodies to Rac1 (top). Cell lysates were also directly subjected to immunoblot analysis with antibodies to Vav2 (Babraham Co.), FLAG, and β-actin. The quantitative results for an active form of Rac from the PAK-RBD assay are shown in graph form (bottom).