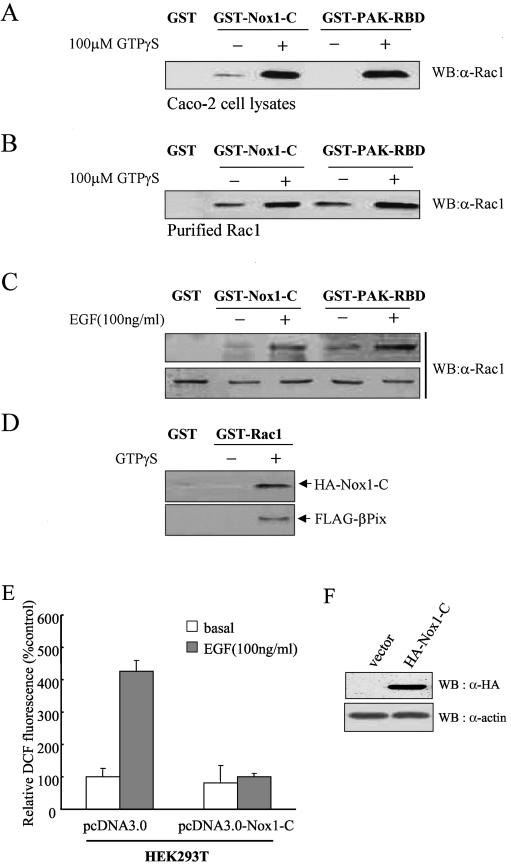
FIG. 6.
Direct interaction of Rac1 with Nox1. A Caco-2 cell lysate (A) or purified Rac1 (1 μg) (B) was incubated first for 10 min at room temperature in the absence or presence of 100 μM GTP-γ-S and then for 3 h at 4°C with bead-conjugated GST, GST-Nox1-C, or GST-PAK-RBD (10 μg) in a final volume of 500 μl of PBS containing 1% Triton X-100 in the continued absence or presence of 100 μM GTP-γ-S. Proteins that bound specifically to the beads as well as to the cell lysate or purified Rac1 were then subjected to immunoblot analysis with antibodies to Rac1. (C) Caco-2 cells were deprived of serum for 16 h and then incubated for 10 min in the absence or presence of EGF (100 ng/ml). Cell lysates were then incubated for 3 h with bead-bound GST, GST-Nox1-C, or GST-PAK-RBD. Proteins retained specifically by the beads were subjected to immunoblot analysis with antibodies to Rac1 (upper panel). Cell lysates were also subjected directly to immunoblot analysis with the same antibodies (lower panel). (D) GST-Rac was incubated first for 10 min at room temperature in the absence or presence of 100 μM GTP-γ-S and then with lysates of HEK293T cells transfected with HA-Nox1-C together with FLAG-βPix for 3 h at 4°C. The beads were then washed with ice-cold cell lysis buffer, and bound proteins were subjected to immunoblot analysis with antibodies to HA or FLAG. (E) HEK293T cells transfected with a vector encoding Nox1-C (pcDNA3.0-HA-Nox1-C) or the corresponding empty vector (pcDNA3.0) were deprived of serum for 16 h and then incubated for 10 min in the absence or presence of EGF (100 ng/ml). The generation of H2O2 was assayed on the basis of DCF fluorescence. Data are expressed as mean fluorescence intensities relative to that for the corresponding unstimulated cells transfected with empty vector and are means ± SE of values from five independent experiments. (F) Cells transfected as for panel E were lysed and subjected to immunoblot analysis with antibodies to HA; filters were reprobed with antibodies to β-actin.