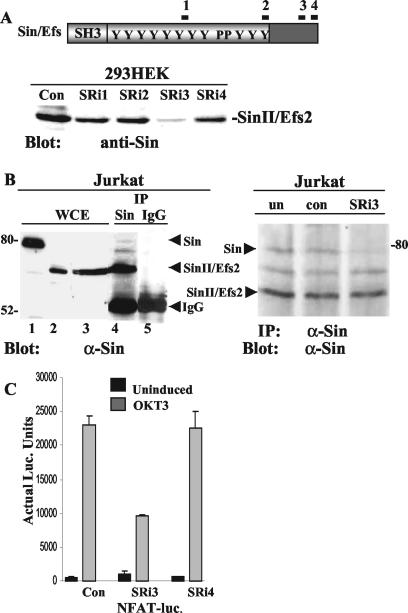
FIG. 7.
Downregulation of Sin expression inhibits TCR-induced transcriptional regulation. (A) A total of 4 × 105 293 HEK cells were transfected with 100 ng of a plasmid expressing human SinII/Efs2 along with 200 ng of Sin-specific or control (Con) siRNA. Thirty-six hours after transfection, cells were harvested and lysed. Whole-cell extracts were normalized for protein content, and proteins were separated by SDS-PAGE. Western blots were probed with Sin-specific antibody, and protein bands were visualized by ECL. (B) Jurkat Tag-cell extracts were immunoprecipitated with Sin-specific or control antibody, and Western blots of immune complexes separated by SDS-PAGE were probed with anti-Sin (α-Sin) antibody (left panel). Whole-cell extracts (WCE) from Jurkat cells overexpressing Sin (lane 1), SinII/Efs2 (lane 2), or untransfected cells (lane 3) were included as controls. Nine times more protein was loaded onto lane 3 compared to lane 2 to reveal endogenous protein levels of Sin isoforms. For downregulation of endogenous Sin expression, 4 × 105 Jurkat Tag cells/well in multiple wells were either left untransfected (un) or transfected with 400 ng of control (con) or Sin-specific siRNA. After 24 to 36 h, cells were lysed and extracts were immunoprecipitated with anti-Sin antibody. Western blots of immune complexes were probed with the same antibody and protein bands visualized by ECL (right panel). One of two independent experiments is shown. IP, immunoprecipitation. (C) Jurkat Tag cells (4 × 105) transfected with 400 ng of control or Sin-specific siRNA, 40 ng of NFAT-firefly-luciferase, and 1 ng of pRL-TK-Renilla-luciferase plasmid DNA. Twenty-four hours after transfection, cells were induced with 1 μg of OKT3/well for 8 h, cells were harvested and lysed, and luciferase (luc.) assays were performed as described in the legend to Fig. 4 and Materials and Methods. Results from one of at least four independent experiments is shown.