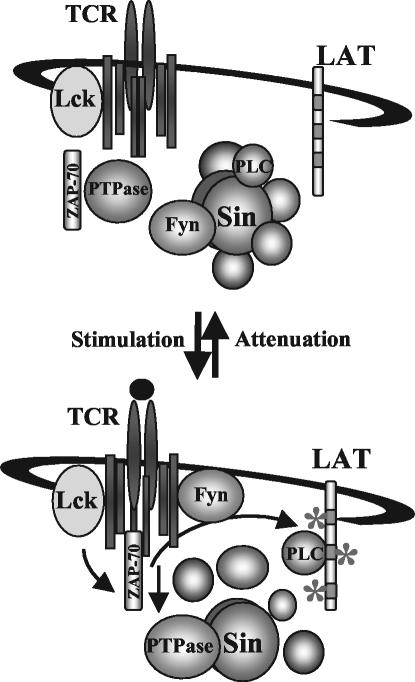
FIG. 8.
Model for Sin-mediated regulation of TCR signaling. In resting T cells, Sin is constitutively phosphorylated by Fyn and bound to PLC-γ and other proteins. TCR stimulation induces the dephosphorylation of Sin, likely through the action of a tyrosine phosphatase, which correlates with the rapid and transient release of associated substrates including Fyn and PLC-γ. Fyn and PLC-γ bind to their respective targets to mediate TCR signaling. Attenuation of TCR signaling 10 min after stimulation results in the reassociation of Fyn and PLC-γ with Sin and Sin phosphorylation, reformation of the inactive complex, and return to resting conditions.