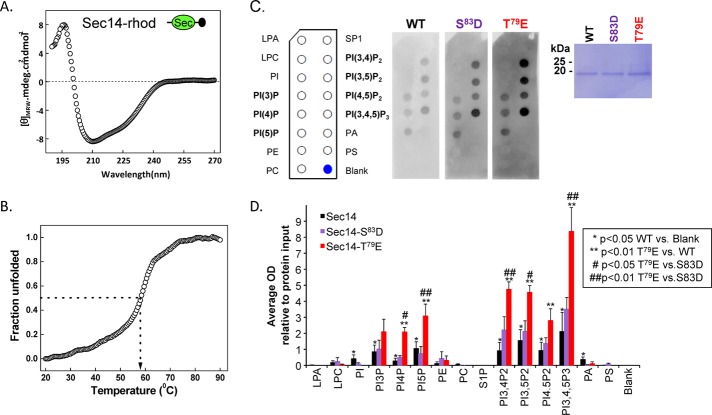
FIGURE 7:
The Sec14 domain of Kalirin interacts with phosphoinositides. (A) The circular dichroism spectrum (average of six determinations) of purified rhodopsin-tagged KalSec14 (inset illustrates location of epitope tag [black circle]) was evaluated from 190 to 270 nm; the spectrum obtained for KalSec14/T79E was indistinguishable (not shown). (B) Cooperative unfolding was observed in a thermal denaturation study of KalSec14; the transition temperature observed was 58 ± 1°C for WT-KalSec14 and 57 ± 1°C for KalSec14/T79E. (C) Purified WT-KalSec14, KalSec14/T79E, and KalSec14/S83D (1 μg/ml; inset: Coomassie-stained membrane demonstrating the purity of the recombinant proteins) were incubated with PIP strips as described in Materials and Methods; bound protein was visualized using antibody to the rhodopsin epitope tag. The lipids on the PIP strips are identified. (D) Data from five experiments were quantified; after background subtraction and normalization to the binding of WT-KalSec14 to PI(3,4,5)P3, data were averaged; error bars represent SE of the mean. No significant binding was observed to lysophosphatidic acid (LPA), lysophosphatidylcholine (LPC), phosphatidylethanolamine (PE), phosphatidylcholine (PC), sphingosine-1-phosphate (SP1), or phosphatidylserine (PS). WT-Sec14 exhibited significant binding to each of the phosphatidylinositides (*p < 0.05 WT vs. blank; Holm–Sidak multiple-comparisons test). Compared to WT-Sec14 and Sec14/S83D, Sec14/T79E exhibited enhanced binding to each phosphorylated phosphatidylinositide except PI(3)P (**,##p < 0.01 Sec14/T79E vs. WT and Sec14/S83D, respectively; Holm–Sidak multiple-comparisons test). After background subtraction and averaging, data for Sec14/S83D did not differ significantly from WT.