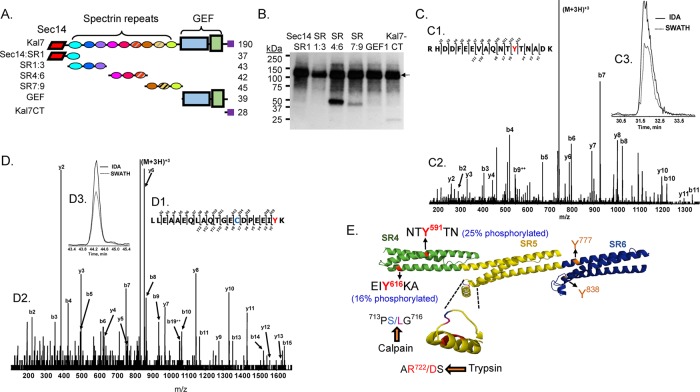
FIGURE 9:
Phosphorylation of Kal SR4:6 by Abl1. (A) The purified fragments of Kalirin (0.5 μM) shown in the diagram were each exposed to recombinant Abl1 (10 nM) along with 5 μM ATP and 0.75 μCi [32P-γ]ATP at 32°C for 30 min. (B) The reactions were stopped by boiling each sample into Laemmli sample buffer; after SDS–PAGE and transfer to a polyvinylidene fluoride membrane, phosphorylated proteins were visualized by autoradiography (3-h exposure). In addition to autophosphorylated Abl1 (135 kDa; arrow), KalSR4:6 was phosphorylated (band at ∼50 kDa); less extensive phosphorylation of KalSR7:9 and Kal7-CT was also detectable. (C, D) The reaction was scaled up as described in Materials and Methods, and phosphorylated SR4:6 recovered from a silver-stained 4–15% acrylamide gel was subjected to in-gel digestion with trypsin, followed by LC-MS/MS identification of phosphorylated Tyr residues. Sites are identified using the NP_114451.2 numbering scheme for Kalrn. MS/MS fragmentation patterns for the Tyr-591 (C) and Tyr-616 (D) peptides are shown. Manual verification and the site of Tyr modification (red Y) are illustrated in C1 and D1. The b- and y-ion series peak assignments are shown (C2, D2). Extracted ion chromatograms of the parent ion (M+2H)2+ for the IDA and SWATH runs were retention time aligned (C3 and D3). Note that the Cys (blue) in the Tyr-616 peptide was acrylamide gel modified (propionamide). (E) Model illustrates the location of the four Tyr residues in KalSR4:6. For each Tyr, percentage phosphotyrosine (shown in parentheses) was calculated relative to the sum of all of the peptides containing that Tyr residue (phosphorylated or not). The protease-sensitive insert in the B-C loop of SR5 (inset) contains the only predicted calpain-cleavage site (Vishwanatha et al., 2012)