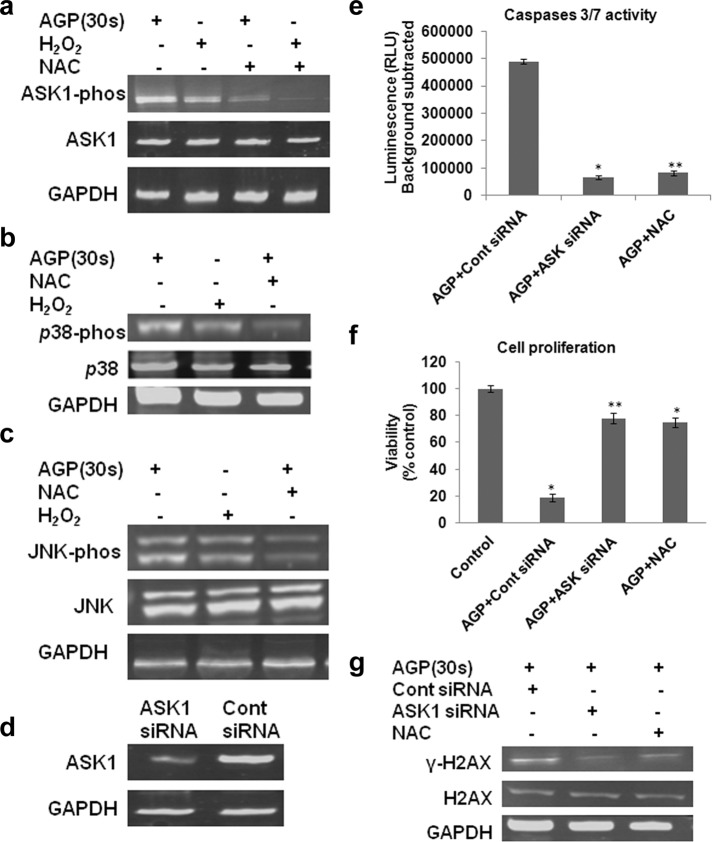
FIGURE 5:
AGP stimulates ASK1-mediated p38, and JNK activation is ROS dependent and reversed with NAC. (a–c) Mel007 cancer cells were treated with AGP or H2O2 with or without pretreatment with NAC. Western blot analysis was performed on cell lysate using different antibodies as indicated (p-ASK1, S83 phosphospecific antibody for ASK1; p-JNK, phosphospecific antibody for JNK; p-p38, phosphospecific antibody for p-38). GAPDH antibody was used as a loading control. (d) Knockdown of ASK1 leads to decreased activation effect of AGP in melanoma cells. Mel007 cancer cells were transfected with small interfering RNA (siRNA) for ASK1 or control siRNA. The cells were analyzed 36–48 h after transfection to determine ASK1 protein levels. GAPDH was used as protein loading control. (e, f) At 24 h after treatment of ASK1 siRNA or control siRNA, Mel007 cells were treated with AGP (30 s) with or without pretreatment with NAC. Caspase 3/7 activity was measured by Caspase-Glo 3/7 assay, and cell viability was measured by cell titer nonradioactive cell proliferation assay. All values are mean ± SD of three independent experiments performed in triplicate. *p ≤ 0.01, **p ≤ 0.001; ANOVA. (g) 2At 4 h posttreatment of ASK1 siRNA or control siRNA, Mel007 cells were treated with AGP (30 s) with or without pretreatment with NAC. The effects of AGP on stress response targets were determined by Western blot analysis of H2AX and γ-H2AX protein levels in cancer cells. GAPDH expression was used as a loading control. In all experiments, control cells were mock treated with He gas flow only.