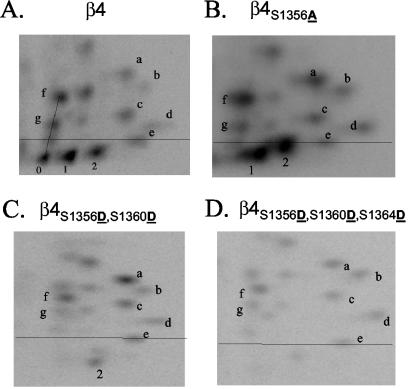
FIG. 2.
Peptide mapping analysis of phosphorylated wild-type and mutant β4 integrin. Excised bands containing 32P-radiolabeled wild-type β4 from HaCat cells (A) or mutated β4 from COS-7 transfectants (B to D) were digested using trypsin, and the peptides were resolved in two dimensions using a combination of TLE and TLC and exposed to a phosphor screen. The phosphopeptides of interest for this study (pp0, pp1, and pp2) are located below a line defined by a control dye. Notice the disappearance of pp0 and both an increase and relocation of pp1 and pp2 to more hydrophobic (higher) positions in β4S1356A (B); the disappearance of pp0 and pp1, as well as the relocation of pp2 to a more hydrophilic (lower) position in the double mutant β4S1356D S1360D (C); and the disappearance of pp0, pp1, and pp2 in the triple mutant β4S1356D S1360D S1364D (D).