Scheme 1.
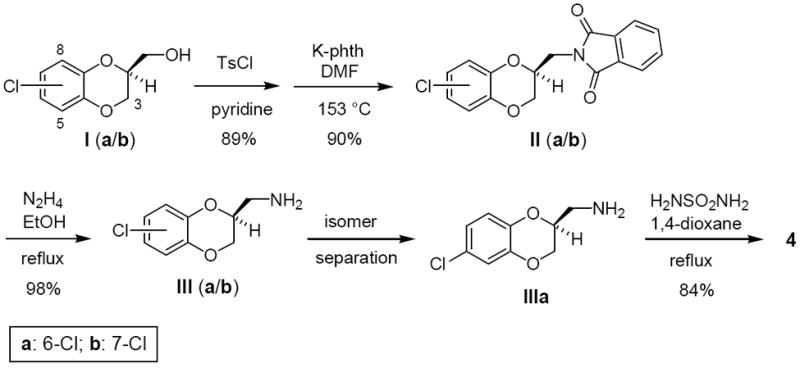
Synthesis of 4.a
a. Alcohols Ia and Ib were prepared as a mixture of regioisomers (~3:1) by following a reported enantioselective synthesis (ref 12). The ratio of IIIa:IIIb was ~3:1. Amine llla was purified as the HCl salt. Ts, p-toluenesulfonyl; phth, phthalimide anion.
