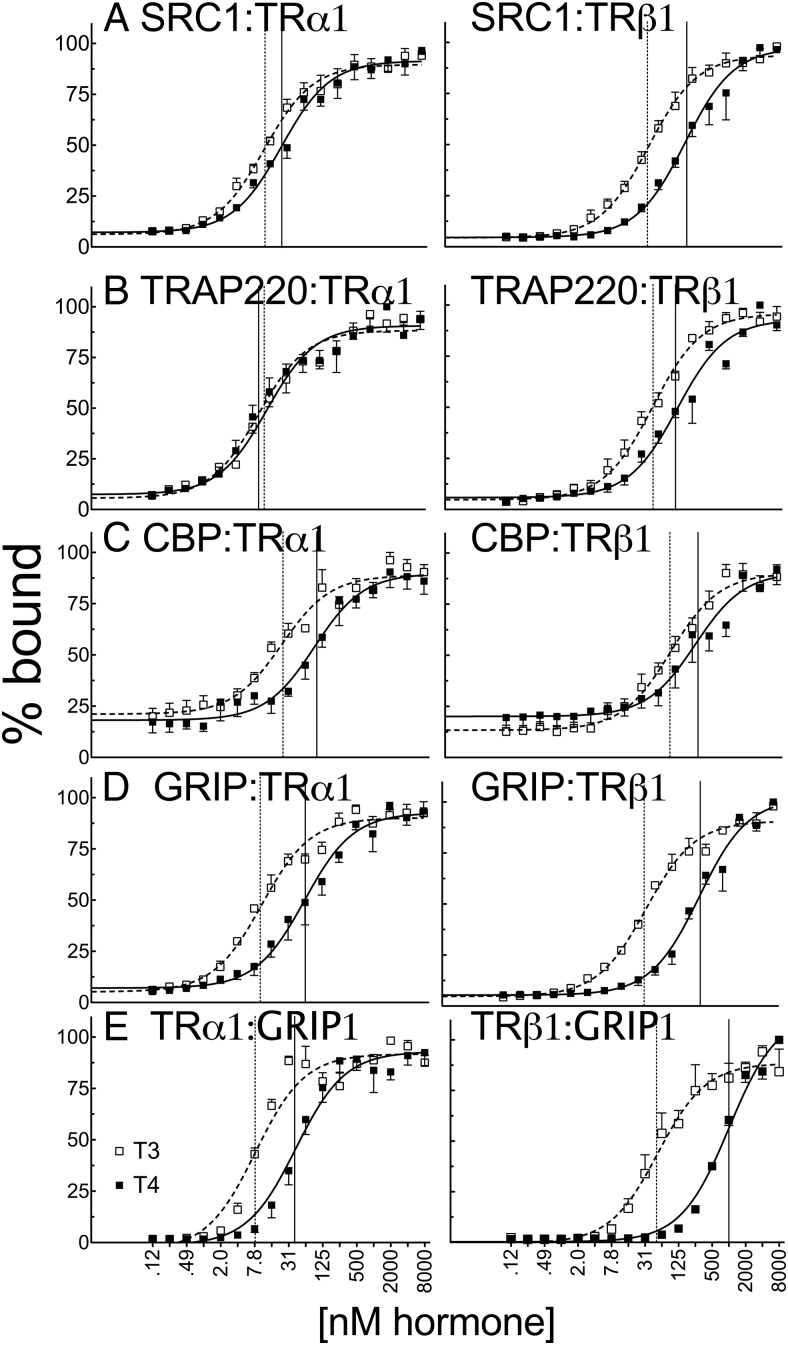
Figure 6.
T4 recruits certain coactivators to TRα1 with potency that was nearly equal to that of T3 in vitro. Full-length in vitro–transcribed and translated, 35S-labeled human TRα1 or TRβ1 were incubated with glutathione-agarose bead immobilized GST fusions of coactivator proteins (LXXLL receptor interaction motifs) indicated within each panel: SRC1 (amino acids 568–891) (A), TRAP220 (486–723) (B), CBP (1–451) (C), and GRIP (544–767) (D). Incubations were conducted in the presence of the indicated T3 or T4 concentration. The immobilized GST constructs were washed, and the nuclear receptors remaining bound to each construct were eluted. The resulting coactivator-TR complexes were characterized by SDS-PAGE and phosphorimager analysis and plotted as the percent maximum binding for each coactivator. Error bars indicate SEs of at least 3 replicate experiments. E, Reverse experiment was conducted with full-length in vitro–transcribed and translated 35S-labeled GRIP1 with immobilized full-length GST-TRα1 or GST-TRβ1, with vehicle alone or the indicated T3 or T4 hormone concentration. Vertical dashed and solid lines represent EC50s T3 and T4, respectively.