Figure 3.
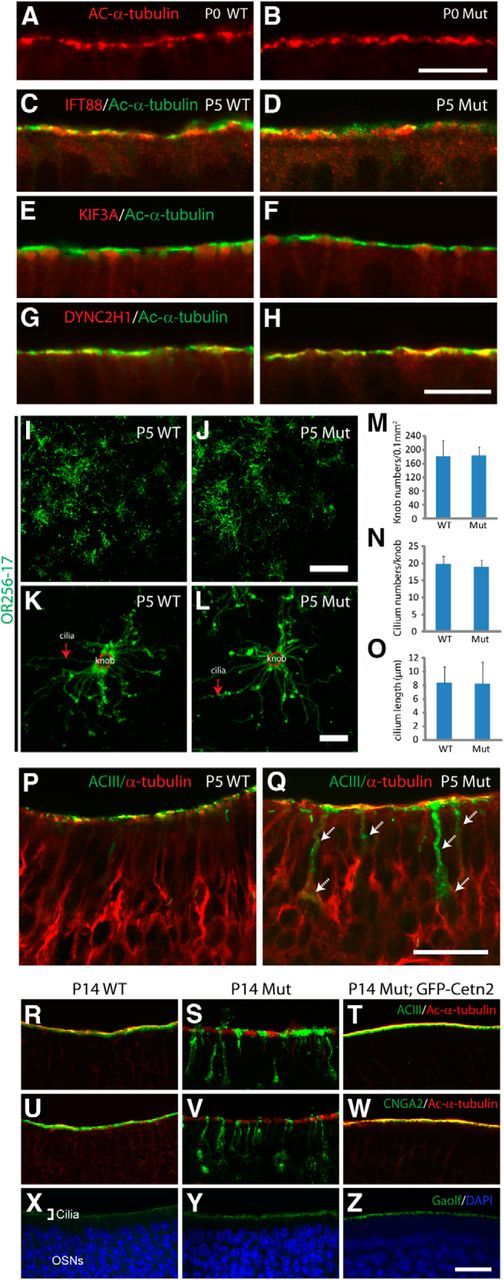
Mislocalization of ACIII and CNGA2 occurs before ciliary loss in Cetn2 mutant. A, B, Ac-α-tubulin immunolabeling of P0 WT and mutant OE. C–H, Colabeling of IFT88 (C, D), KIF3A (E, F), and DYNC2H1 (G, H; all red) with Ac-α-tubulin (green) in P5 WT and mutant OE, showing comparable patterns. I–L, OR256-17 staining of P5 WT and mutant whole-mount OE at low- (I, J) and high-magnification (K, L). M–O, No statistical difference of knob density (M), cilia per knob (N), or average ciliary length (O) was observed. Data are mean ± SD (one-way ANOVA, p > 0.8, n ≥ 45 OSNs from 3 animals per group). P, Q, Colabeling of P5 turbinate I OE. ACIII (green) colocalizes with α-tubulin (red) at the cilium layer of WT (P), but is partially mislocalized to the dendrite and cell body (arrows) in mutant (Q). Mutant olfactory cilia appear intact at this stage. R–T, Double-immunofluorescence of ACIII (green) and Ac-α-tubulin (red) in P14 WT (R), Cetn2 mutant (S), and Cetn2 Mut;GFP-Cetn2 (T) OE. O–Q, Double-staining of CNGA2 (green) and Ac-α-tubulin (red) in P14 WT (U), Cetn2 mutants (V), and Cetn2 Mut;GFP-CETN2 (W) OE. X–Z, Immunofluorescence of Gαolf in OE. Gαolf localizes in cilia layer of P14 WT (X), mutant (Y), and Cetn2 Mut;GFP-Cetn2 (Z) OE. Please note the endogenous GFP-CETN2 signal was quenched in T, W, Z by boiling in 10 mm sodium citrate, pH6.0, for 20 min before staining. Scale bars: A, B, I, J, P, Q, 10 μm; K, L, 5 μm; R–Z, 30 μm.
